Abstract
Benign childhood epilepsy with centrotemporal spikes (BECTS) has been investigated through EEG–fMRI with the aim of localizing the generators of the epileptic activity, revealing, in most cases, the activation of the sensory–motor cortex ipsilateral to the centrotemporal spikes (CTS). In this case report, we investigated the brain circuits hemodynamically involved by CTS recorded during wakefulness and sleep in one boy with CTS and a language disorder but without epilepsy. For this purpose, the patient underwent EEG–fMRI coregistration. During the “awake session”, fMRI analysis of right-sided CTS showed increments of BOLD signal in the bilateral sensory–motor cortex. During the “sleep session”, BOLD increments related to right-sided CTS were observed in a widespread bilateral cortical–subcortical network involving the thalamus, basal ganglia, sensory–motor cortex, perisylvian cortex, and cerebellum.
In this patient, who fulfilled neither the diagnostic criteria for BECTS nor that for electrical status epilepticus in sleep (ESES), the transition from wakefulness to sleep was related to the involvement of a widespread cortical–subcortical network related to CTS. In particular, the involvement of a thalamic–perisylvian neural network similar to the one previously observed in patients with ESES suggests a common sleep-related network dysfunction even in cases with milder phenotypes without seizures. This finding, if confirmed in a larger cohort of patients, could have relevant therapeutic implication.
Abbreviations: CTS, centrotemporal spikes; BECTS, benign epilepsy with centrotemporal spikes; BOLD, blood oxygen level dependent; ESI, EEG source imaging; ESES, electrical status epilepticus in sleep
Keywords: EEG–fMRI, BECTS, ESES, Thalamus, Sleep
1. Introduction
Benign childhood epilepsy with centrotemporal spikes (BECTS) is an idiopathic focal epilepsy characterized by distinctive interictal EEG paroxysms over rolandic regions, age-dependent onset, and benign course [1]. The rolandic or centrotemporal spikes (CTS) show characteristic waveform features and are significantly enhanced during NREM sleep [2]. The increase of CTS frequency during slow-wave sleep might cause the worsening in language and executive functions, as observed in patients with atypical BECTS and in electrical status epilepticus during sleep (ESES) [3]. In this respect, the study of the brain networks involved by CTS while awake and asleep in the same patient is an intriguing, still open question. It is reasonable to hypothesize that different networks might be triggered by CTS during sleep, in relation to changes in the patient's level of vigilance. We expect that CTS in sleep may involve extra sensory–motor networks and especially subcortical, namely thalamic, structures [4].
To address these issues, we present the case of a 13-year-old boy with moderate language impairment and CTS who underwent EEG–fMRI coregistration and EEG source imaging (ESI) during both awake and asleep periods. Notably, the patient came to our attention because he had language disorder/learning difficulties, and, at the time of the study, no overt seizure occurred.
2. Patient and methods
A 13-year-old right-handed boy was referred to our center for investigating school difficulties. His past medical history, including birth and development milestones, was unremarkable. The patient's family history shows three paternal cousins affected by a benign form of epilepsy not otherwise specified. At the neuropsychological assessment, a discrepancy in the linguistic (mildly deficient) and nonlinguistic functions (normal) was found, and moderate-learning difficulties in reading, writing, and calculation emerged.
The patient underwent scalp EEG while awake and asleep, demonstrating the presence of CTS occurring independently in the right and left hemispheres which were significantly increased during slow-wave sleep (Fig. 1A–B). A complete overnight video-EEG recording confirmed an increase in CTS frequency during non-REM sleep but without reaching the criteria for ESES (spike index > 85%).
Fig. 1.
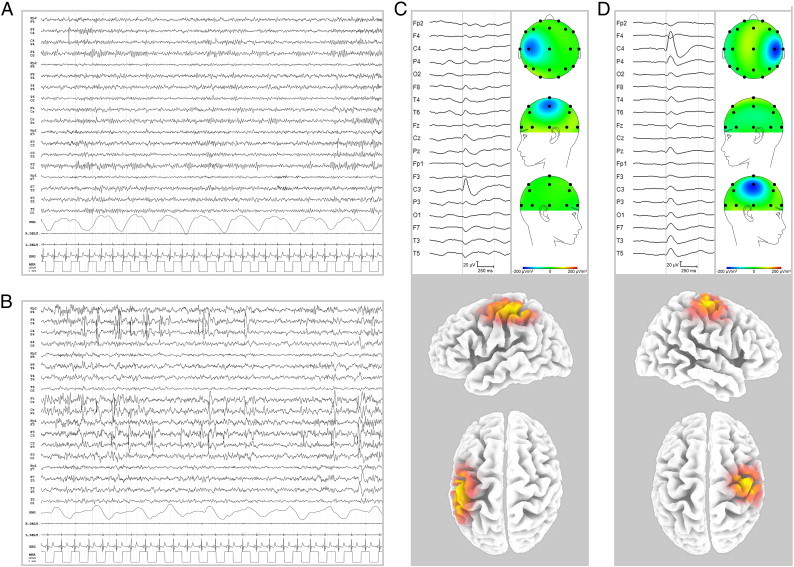
Patient's EEG trace while awake and asleep and ESI results. Panel A: representative page of the EEG during wakefulness. Rare independently and isolated CTS from the left and right hemispheres are evident. Panel B: representative page of the EEG recorded during sleep (phase 2 NREM). Note the increase in CTS frequency compared to wakefulness. CTS appear isolated or in brief discharges, synchronous or asynchronous over the two hemispheres. Sleep spindles and vertex spike are evident. The EEG traces are shown in bipolar montage. Panel C: spike averaging and topographic map related to the left CTS events (top image); ESI (bottom image) related to the left CTS demonstrates a main source over the left sensory–motor cortex. The electric potential field used by the sLORETA software was computed using the boundary element method applied to the MNI152 template. The MNI brain volume was scanned at 5-mm resolution obtaining a source space of 6239 cortical gray matter voxels. The sLORETA algorithm returns the current density measure (in [A/m2]) for each of the cortical voxels, indicating the MNI coordinates of the best fit, i.e., the voxel with the maximum current density value. Panel D: Spike averaging and topographic map related to the right CTS events (top image); ESI (bottom image) related to the right CTS demonstrates a main source over the right sensory–motor cortex.
2.1. EEG–fMRI acquisition
Centrotemporal spikes were recorded in the patient, who was sleep-deprived from the previous night, in the late afternoon. Scalp EEG was recorded by means of a 32-channel MRI-compatible EEG recording system (Micromed, Italy). A simultaneously recorded video during the EEG–fMRI acquisition allowed checking patient's behavior and changes in vigilance states. Functional magnetic resonance imaging data (200 volumes, 30 axial slices, TR/TE = 3000/50 ms) were acquired over three 10-min sessions with simultaneous EEG recording using a Philips Intera System 3T. A high-resolution T1-weighted anatomical image was obtained for anatomical reference (170 sagittal slices, TR/TE = 9.9/4.6 ms).
The Human Ethic Committee of the University of Modena and Reggio Emilia approved the study, and written consent was obtained.
2.2. EEG and fMRI analysis
Off-line analysis of the EEG was performed by means of the BrainVision Analyzer 2.0 software (Brain Products, Munich, Germany). The detection of sleep stages was defined based on the presence of sleep spindles and K-complexes and confirmed by the video record. Functional magnetic resonance imaging data were preprocessed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Two separate analyses were performed: the first related to CTS during wakefulness and the second related to CTS during the sleep phase. Centrotemporal spikes were visually marked and served as onsets for a general linear model (GLM) convolved with the standard hemodynamic response function (HRF), its temporal (TD) and dispersion derivatives (DD). For the sleep-related fMRI maps, K-complex and spindles were visually identified and marked according to the criteria of Rechtschaffen and Kales [5]. The onset of K-complexes and spindles was included in the general linear model (GLM) as a separate regressor. Functional magnetic resonance imaging scan time series realignment parameters were used to model the nuisance effects of motion. One-tailed t-test was applied to test for regional BOLD increases or decreases in relationship to the CTS. The computed SPM{T} was thresholded at P < 0.05 (FWE corrected). Results were superimposed onto the individual high-resolution T1-weighted images normalized into the MNI space.
EEG source imaging (ESI) analysis of CTS recorded during scanning was performed with 3D sLORETA (standardized low resolution brain electromagnetic tomography). The equivalent source current density for each spike was estimated using component topographies as input data.
3. Results
During the first 10-min session, the patient was awake and 69 CTS (6.9 spikes/min) were identified: 47 from the right hemisphere and 22 from the left. During the second and third sessions, the patient fell asleep, reaching phase two of NREM sleep, and 249 CTS (12.5 spikes/min) were observed: 191 from the right hemisphere and 58 from the left.
3.1. EEG source imaging
Spike averaging and localization results of CTS during wakefulness and sleep are displayed in Fig. 1C–D. EEG source imaging analysis showed the involvement of the sensory–motor cortex ipsilateral to CTS, both during awake and asleep periods.
3.2. EEG–fMRI study
During the “awake session”, fMRI analysis of right-sided CTS showed increments of BOLD signal in the bilateral sensory–motor cortex (Fig. 2, left panel). No BOLD signal changes were detected for left-sided CTS. During the “sleep session”, BOLD increments related to right-sided CTS were observed in a widespread bilateral cortical–subcortical network involving the thalamus, basal ganglia, sensory–motor cortex, perisylvian cortex, paracentral lobule, anterior cingulated cortex, and cerebellum (Fig. 2, right panel). The same results were obtained in relation to the left-sided CTS, although with less statistical power (not shown). No decrements in BOLD signal were detected in both sessions.
Fig. 2.
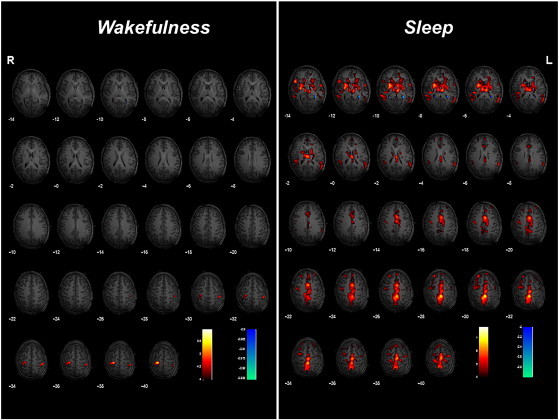
EEG–fMRI results. Left panel. Wakefulness: A color-coded overlay of SPM{T} (red: positive BOLD response; blue: negative BOLD response)-HRF related (P < 0.05 corrected for multiple comparison) onto the patient's T1 slice overlay showing BOLD signal increases related to right CTS in the bilateral sensory–motor cortex. BOLD signal changes for left-sided CTS and a decrease in clusters for both right and left CTS were not detected. Right panel. “Sleep”: A color-coded overlay of SPM{T} (red: positive BOLD response; blue: negative BOLD response)-HRF related (P < 0.05 corrected for multiple comparison) onto the patient's T1 slice overlay showing BOLD signal increases related to right CTS involving the bilateral thalamus, basal ganglia, sensory–motor cortex, perisylvian cortex, paracentral lobule, cingulated cortex, and cerebellum. fMRI data analysis regarding TD and DD did not demonstrate any significant hemodynamic changes both in the awake and asleep sessions. No BOLD signal decreases were detected.
4. Discussion
The main finding of the study is that EEG–fMRI data analysis of CTS recorded from the same patient while awake and asleep revealed different hemodynamic networks. In particular, sleep-related fMRI maps showed the involvement of a wide network including the thalamus. Conversely, the hemodynamic changes observed during the “awake state” covered the sensory–motor cortex, concordant with ESI results and with previous EEG–fMRI studies. Indeed, fMRI data analysis in typical BECTS revealed, in most cases, a cortical activation limited to the sensory–motor cortex ipsilateral to CTS [6–9].
Our data confirm the involvement of thalamocortical neurons in the promotion of CTS during NREM sleep. These findings are not surprising if we consider that the facilitation of CTS during NREM sleep is due to two different thalamic mechanisms of synchronization that lead to the appearance of spindles and delta waves on the EEG [4]. The activating properties of these two mechanisms on CTS generations have been demonstrated through animal experimental data [10] and also in humans by means of EEG spectral analysis [11]. In particular, a high significant correlation between CTS and sigma activity has been observed in BECTS, suggesting that CTS are sensitive to the promoting action of the spindle-generating mechanisms [4,11].
Previous EEG–fMRI studies in patients classified as having purely BECTS did not show the cortical–subcortical network observed in our case while asleep [6–9]. On the contrary, in our patient, we observed fMRI activation of the thalamus and of the bilateral perisylvian regions, including the first temporal gyrus, suggesting that these areas could be functionally altered. Notably, this finding could be related to the clinical picture of the patient who could not fulfill the clear diagnostic criteria for BECTS or for ESES, falling into the category of patients with CTS and a learning/language disorder.
Interestingly, these results are similar to the ones observed by EEG–fMRI analysis in patients with ESES [12], supporting the existence of a common sleep-related network dysfunction even in cases with milder phenotypes.
Recently, the term “intermediate epilepsy-aphasia disorder” has been proposed to classify those patients showing a combination of abnormal cognitive development (predominantly affecting language function), with or without clinical seizures, and sleep activation of focal epileptiform discharges not fulfilling the electroclinical criteria for BECTS, Landau–Kleffner syndrome, or ESES but considered to have a place within the spectrum [13]. In this context, the possibility of demonstrating, by means of fMRI, a network dysfunction in patients with CTS and moderate cognitive impairment, like our patient, may represent a chance to identify these patients in early stages. Our work hence provides evidence for a potential diagnostic value of the EEG–fMRI studies as a marker of atypical BECTS.
Finally, the information obtained by the EEG–fMRI technique adds value to the theory that idiopathic focal epilepsies result from a dysfunction of distributed neuronal systems extending beyond a focal cortical seed but involving thalamic and thalamocortical circuits that show a hyperexcitable condition as a whole [14]. Of course, further studies on a larger cohort of patients are needed to confirm this hypothesis.
Acknowledgments and funding
Author RA was supported by a PhD bursary from the University of Modena and Reggio Emilia. Author AEV was supported by a research bursary from the grant “Ricerca Internazionale 2010” of “Fondazione Cassa di Risparmio di Modena”.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for classification of epilepsies, epileptic syndromes and related disorders. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner C., Graf M., Doppelbauer A., Serles W., Lindinger G., Olbrich A. The functional organization of the interictal spike complex in benign rolandic epilepsy. Epilepsia. 1996;37:1164–1174. doi: 10.1111/j.1528-1157.1996.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 3.Tassinari C.A., Cantalupo G., Dalla Bernardina B., Darra F., Bureau M., Cirelli C. Encephalopathy related to status epilepticus during slow sleep (ESES) including Landau–Kleffner syndrome. In: Bureau M., Genton P., Dravet C., Delgado-Escueta A., Tassinari C.A., Thomas P., editors. Epileptic syndromes in infancy, childhood and adolescence. 5th ed. John Libbey Eurotext Ltd.; Montrouge, France: 2012. pp. 255–275. [Google Scholar]
- 4.Nobili L., Baglietto M.G., Beelke M., De Carli F., De Negri E., Gaggero R. Distribution of epileptiform discharges during nREM sleep in the CSWSS syndrome: relationship with sigma and delta activities. Epilepsy Res. 2001;44:119–128. doi: 10.1016/s0920-1211(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 5.Rechtschaffen A., Kales A. Terminology. In: Rechtschaffen A., Kales A., editors. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. BIS/BRI; Los Angeles: 1968. pp. 1–2. [Google Scholar]
- 6.Archer J.S., Briellman R.S., Abbott D.F., Syngeniotis A., Wellard R.M., Jackson G.D. Benign epilepsy with centro-temporal spikes: spike triggered fMRI shows somato-sensory cortex activity. Epilepsia. 2003;44:200–204. doi: 10.1046/j.1528-1157.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- 7.Boor S., Vucurevic G., Pfleiderer C., Stoeter P., Kutschke G., Boor R. EEG-related functional MRI in benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2003;44:688–692. doi: 10.1046/j.1528-1157.2003.27802.x. [DOI] [PubMed] [Google Scholar]
- 8.Boor R., Jacobs J., Hinzmann A., Bauermann T., Scherg M., Boor S. Combined spike-related functional MRI and multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clin Neurophysiol. 2007;118:901–909. doi: 10.1016/j.clinph.2006.11.272. [DOI] [PubMed] [Google Scholar]
- 9.Masterton R.A.J., Harvey A.S., Archer J.S., Lillywhite L.M., Abbott D.A., Scheffer I.E. Focal epileptiform spikes do not show a canonical BOLD response in patients with benign rolandic epilepsy (BECTS) Neuroimage. 2010;51:252–260. doi: 10.1016/j.neuroimage.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M., Contreras D., Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 1994;17:199–208. doi: 10.1016/0166-2236(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 11.Nobili L., Ferrillo F., Baglietto M.G., Beelke M., De Carli F., De Negri E. Relationship of sleep interictal epileptiform discharges to sigma activity (12–16 Hz) in benign epilepsy of childhood with rolandic spikes. Clin Neurophysiol. 1999;110:39–46. doi: 10.1016/s0168-5597(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Siniatchkin M., Groening M., Moehring J., Moeller F., Boor R., Brodbeck V. Neuronal networks in children with continuous spikes and waves during slow sleep. Brain. 2010;133:2798–2813. doi: 10.1093/brain/awq183. [DOI] [PubMed] [Google Scholar]
- 13.Tsai M.H., Vears D.V., Turner S.J., Smith R.L., Berkovic S.L., Sadleir L.G. Clinical genetic study of the epilepsy-aphasia spectrum. Epilepsia. 2013;54:280–287. doi: 10.1111/epi.12065. [DOI] [PubMed] [Google Scholar]
- 14.Avanzini G., Manganotti P., Meletti S., Moshé S.L., Panzica F., Wolf P. The system epilepsies: a pathophysiological hypothesis. Epilepsia. 2012;53:771–778. doi: 10.1111/j.1528-1167.2012.03462.x. [DOI] [PubMed] [Google Scholar]


