Abstract
In ictal psychosis with complex visual hallucinations (VHs), widespread functional changes of cortical networks have been suggested. We describe the clinical and EEG findings of a patient with bipolar disorder who manifested complex VHs associated with intense emotional symptoms caused by frontal epileptic seizures. This description highlights the challenges of diagnosing the epileptic nature of new psychotic phenomena in patients with previous psychiatric disorders and shines light into the role of the frontal cortex in the genesis of complex VHs.
Keywords: Visual hallucinations, Ictal psychosis, Frontal epileptic seizures, Bipolar disorder
1. Introduction
Complex visual hallucinations (VHs) consist of recurrent and well-formed vivid images which can be accompanied by intense emotions or delusions. Visual hallucinations are caused by neuronal functional perturbation not only in the association visual cortex [1] but also in the limbic regions, which are central to the genesis of psychosis, and in the frontal and parietal regions connected with the limbic system [2].
In partial epilepsy, a localized neuronal discharge mainly in the temporal regions can cause subjective symptoms such as ictal psychotic phenomena. However, in epileptic complex hallucinatory phenomena, more widespread changes of cortical networks have been reported, with involvement of the frontal and parietal cortex via a release of the association visual cortex [1,3].
We describe here a patient with a history of bipolar disorder who had frontal partial epileptic seizures manifesting with ictal psychosis characterized by complex VHs and intense fear and disgust. The challenges of diagnosing the epileptic nature of new psychotic manifestations in psychiatric diseases are discussed.
2. Case report
A 75-year-old man was brought to the emergency room of the University Hospital of Padua because he presented with a transient confusional state with an inability to speak while maintaining an open mouth and producing simple vocalizations. During this episode, he tried to cover the face of his wife with a shirt. These symptoms lasted a few minutes, and he complained afterward of having had an unpleasant sensation of a foreign body in his throat, associated with agitation, disgust, and fear. Upon arrival in the hospital, he was treated with 4-mg lorazepam i.v. because an anxiety disorder such as a panic attack was suspected, and he was later discharged. A similar episode occurred a few hours later, and the patient was therefore admitted to the neurology ward.
The patient had a long history of depression and was diagnosed with a bipolar disorder almost ten years earlier. He had been on duloxetine (60 mg/day), quetiapine (125 mg/day), and lorazepam (1 mg/day) for the past few years, while sodium valproate at the dosage of 750 mg/day was added in the previous month as a mood-stabilizing drug. Four days before admission, he abruptly discontinued the sodium valproate treatment for perceived lack of benefit. On admission, the patient was alert and time- and space-oriented, with normal verbal communication abilities. Neurological examination was normal. Routine blood tests including liver functions, thyroid hormones, and microbiology for viral infections and syphilis were unremarkable.
During the first day, he experienced several stereotyped episodes during which he was moving his hands in an attempt to extract something from his mouth and simultaneously covering witnesses' eyes. During these episodes, he was awake but unresponsive to verbal inquiries and unable to explain what was happening. The episodes lasted a few minutes and were followed by a brief period of confusional state associated with anxiety symptoms and reduced verbal fluency with stuttering. After resolution, he did not show neurological, cognitive, or behavioral disturbances. He could then describe that during these confusional states, he had been experiencing the sensation of seeing a foreign body resembling a twisted animal, like a snake with multiple colors, coming out from his throat and preventing him from speaking. Moreover, he explained that during this visual experience, he had been trying to hide the view of this phenomenon from anyone by covering their eyes with his hands. Similar episodes had the characteristics of complex VHs with associated strong emotional feelings of disgust and occurred with a frequency of 5–7 per day. A psychiatric evaluation diagnosed the psychotic disorder as a complication of depression and suggested an increase in the dosage of quetiapine to 150 mg/day.
During EEG recording, he experienced the same complex VH as described above. Ictal EEG showed the sudden appearance of continuous rhythmic spikes at a frequency of 5 Hz/s localized on the frontal regions bilaterally and lasting 40 s, followed by spikes and spike–waves in the same regions, lasting 9 min (Fig. 1), and abruptly discontinued with transient postictal slowing of rhythms (Fig. 2). Afterward, a return of normal EEG activity was maintained in the interictal phases (Fig. 3). Cerebrospinal fluid investigations excluded infective encephalitis. Brain magnetic resonance imaging showed the presence of mild cortical atrophy mainly in the frontal regions.
Fig. 1.
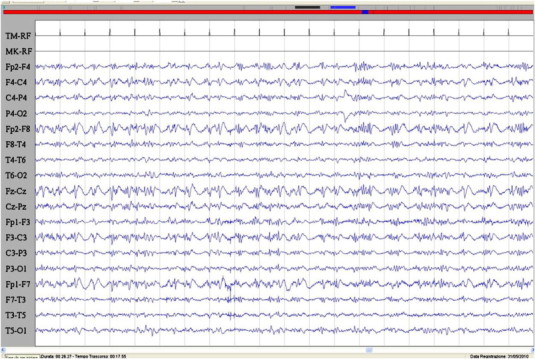
Ictal EEG during the epileptic seizure characterized by acute visual hallucination with fear and speech arrest. A burst of spikes and spike–waves in the frontal regions bilaterally is evident. Sensitivity: 7 μV/mm; TC: 0.1 s; HF: 50.0 Hz.
Fig. 2.
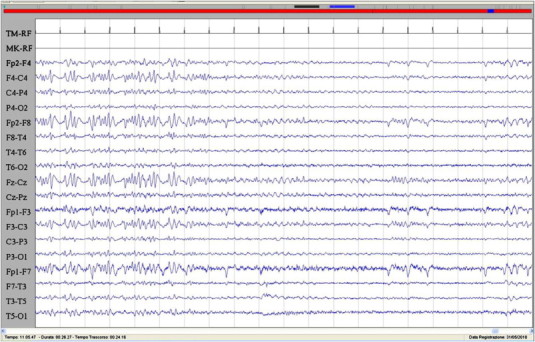
The image showed the abrupt remission of the epileptic activity corresponding to the discontinuation of the hallucinatory phenomenon and the slowing of the EEG activity. Sensitivity: 7 μV/mm; TC: 0.1 s; HF: 50.0 Hz.
Fig. 3.
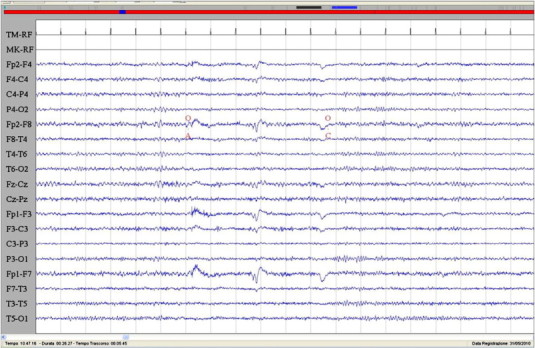
Interictal EEG characterized by normal rhythms with preservation of voltage suppression with eye opening. Sensitivity: 7 μV/mm; TC: 0.1 s; HF: 50.0 Hz.
Remission of the epileptic seizures and the concomitant recurrent VHs was achieved through acute treatment with sodium valproate (starting dosage of 15 mg/kg and then 1 mg/kg/h i.v. for 12 h). In the following week, control EEG monitoring was normal, and epileptic activity was not seen. The patient was discharged from the hospital seizure-free, with sodium valproate (1000 mg/day), lorazepam (2 mg/day), and quetiapine (150 mg/day) treatment. Follow-up clinical and EEG evaluations over the next three years confirmed the lack of recurrence of the epileptic seizures.
3. Discussion and conclusion
We described here a patient with a history of bipolar disorder who presented recurrent and stereotyped episodes of ictal psychosis manifesting as complex VHs associated with intense fear and disgust, secondary to frontal epileptic seizures. This description highlights the challenge of diagnosing the epileptic nature of a new psychotic phenomenon in patients with previous psychiatric disorders.
Elliott and colleagues [4] observe that epileptic hallucinatory states are often indistinguishable from those in the primary psychosis, although these almost always have additional epileptic features such as confusion or altered awareness. In fact, in the case presented here, the epileptic etiology of VHs could have been prediceted by the alteration of awareness and lack of response to verbal inquiries during the ictal phase and the postictal confusional state and agitation. Moreover, neurological symptoms, i.e., reduced verbal fluency and stuttering mimicking an epileptic speech arrest, were present. Although the clinical presentation could have suggested an epileptic origin of these disturbances, the diagnosis of epileptic psychosis was made possible by EEG recording of a prolonged frontal seizure during the experience of complex VHs. The EEG investigation is the most important tool in the differential diagnosis between a primary psychiatric disorder with psychosis and an epileptic psychosis. In epilepsy, ictal psychosis is usually due to the presence of concomitant limbic seizure activity, while interictal psychosis occurs more often in limbic epilepsy in those patients with frequent seizures and a long history of epilepsy.
Epileptic elementary VHs are perceptions of flashing or flickering light, colored light effects, and shining figures possibly in motion and are generated by electrical perturbation of the visual cortex [5]. Complex VHs have a more diffuse neuroanatomical basis than simple hallucinations. Originally, excitation of the association visual cortex in the posterior temporal and parietal regions was considered necessary and sufficient to generate complex VHs [5–7]. Many studies, however, report that complex VHs with a strong emotional component do not occur unless limbic structures are also activated [8]. An involvement of the limbic structures is also supported by the frequent presence of symptoms like fear and panic. Biraben and colleagues [9] suggest that intense ictal fear is related to the activation of a complex neuronal network involving the orbitofrontal cortex, the anterior cingulate cortex, and the limbic regions in the temporal lobe. The finding of a frontal epileptic activity associated with complex VHs described here draws the attention to a partially different cortical network involved in this modality of hallucinatory phenomena [10].
Very few descriptions of patients with frontal lobe epilepsy manifesting with ictal delusions and hallucinations (both auditory and visual) are available in the literature [11,12]. These reports describe pediatric patients with idiopathic epilepsy. The case reported here is, to our knowledge, the first description of an adult patient, without a history of epilepsy, developing frontal lobe epileptic seizures after abrupt discontinuation of sodium valproate. Although withdrawal epileptic seizures due to valproate discontinuation have rarely been described, we suggest that in this patient, the sudden interruption of valproate treatment could have unmasked an epileptic susceptibility [13]. Alternatively, an unreported concomitant poor compliance with other drugs, such as lorazepam, could have contributed to the genesis of the seizures. We suggest that ictal psychosis may develop in patients without epilepsy when favorable conditions occur such as discontinuation of drugs with antiepileptic activity or underlying brain pathology. It is well known that in the psychiatric population the abrupt modification of antiepileptic drugs or benzodiazepines may cause epileptic seizures. Moreover, a cognitive impairment involving frontal lobe cognitive functions and an atrophy of the frontal regions are well established findings in elderly patients with a history of bipolar disorder.
In conclusion, we highlight the importance of considering the epileptic cause of new psychotic phenomena in patients with a psychiatric disorder, particularly when confusional state and fear are associated, and recommend further study of these patients with repeated EEG recordings. Moreover, we confirm previous sporadic evidence that frontal epileptic seizures could underpin ictal complex VHs.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Acknowledgments
This work was supported by research funding from the Department of Neurosciences, University of Padova, Italy.
A.C. was on leave at IRCCS San Camillo Hospital in Venice, Italy, while involved in this study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Manford M., Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819–1840. doi: 10.1093/brain/121.10.1819. [DOI] [PubMed] [Google Scholar]
- 2.Oyebode F. The neurology of psychosis. Med Princ Pract. 2008;17:263–269. doi: 10.1159/000129603. [DOI] [PubMed] [Google Scholar]
- 3.Fish D.R., Gloor P., Quesney F.L., Olivier A. Clinical responses to electrical brain stimulation of the temporal and frontal lobes in patients with epilepsy. Pathophysiological implications. Brain. 1996;119:17–40. doi: 10.1093/brain/116.2.397. [DOI] [PubMed] [Google Scholar]
- 4.Elliott B., Joyce E., Shorvon S. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res. 2009;85:172–186. doi: 10.1016/j.eplepsyres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Elliott B., Eileen J., Shorvon S. Delusions, illusions and hallucinations in epilepsy: 1. Elementary phenomena. Epilepsy Res. 2009;85:162–171. doi: 10.1016/j.eplepsyres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Williamson P.D., Boon P.A., Thadani V.M., Darcey T.M., Spencer D.D., Spencer S.S. Parietal lobe epilepsy: diagnostic considerations and results of surgery. Ann Neurol. 1992;31:193–201. doi: 10.1002/ana.410310210. [DOI] [PubMed] [Google Scholar]
- 7.Penfield W., Perot P. The brain's record of auditory and visual experience. A final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 8.Gloor P., Olivier A., Quesney L.F., Andermann F., Horowitz S. The role of the limbic system in experimental phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- 9.Biraben A., Taussig D., Thomas P., Even C., Vignal J.P., Scarabin J.M. Fear as the main feature of epileptic seizures. J Neurol Neurosurg Psychiatry. 2001;70:186–191. doi: 10.1136/jnnp.70.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fish D.R., Gloor P., Quesney F.L., Olivier A. Clinical responses to electrical brain stimulation of the temporal and frontal lobes in patients with epilepsy: pathophysiological implications. Brain. 1993;16:397–414. doi: 10.1093/brain/116.2.397. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey B.J. Frontal lobe epilepsy presenting as a psychotic disorder with delusions and hallucinations: a case study. Pediatr Neurol. 2006;35:78–81. doi: 10.1017/s1092852900012207. [DOI] [PubMed] [Google Scholar]
- 12.La Vega-Talbot M., Duchowny M., Jayakar P. Orbitofrontal seizures presenting with ictal visual hallucinations and interictal psychosis. Epilepsia. 2007;48:17–19. doi: 10.1016/j.pediatrneurol.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Clarke M.C., Tanskanen A., Huttunen M.O., Clancy M., Cotter D.R., Cannon M. Evidence for shared susceptibility to epilepsy and psychosis: a population-based family study. Biol Psychiatry. 2012;71:836–839. doi: 10.1016/j.biopsych.2012.01.011. [DOI] [PubMed] [Google Scholar]


