Fig. 6. A possible pre-assembly complex of the T2SS Inner Membrane Platform.
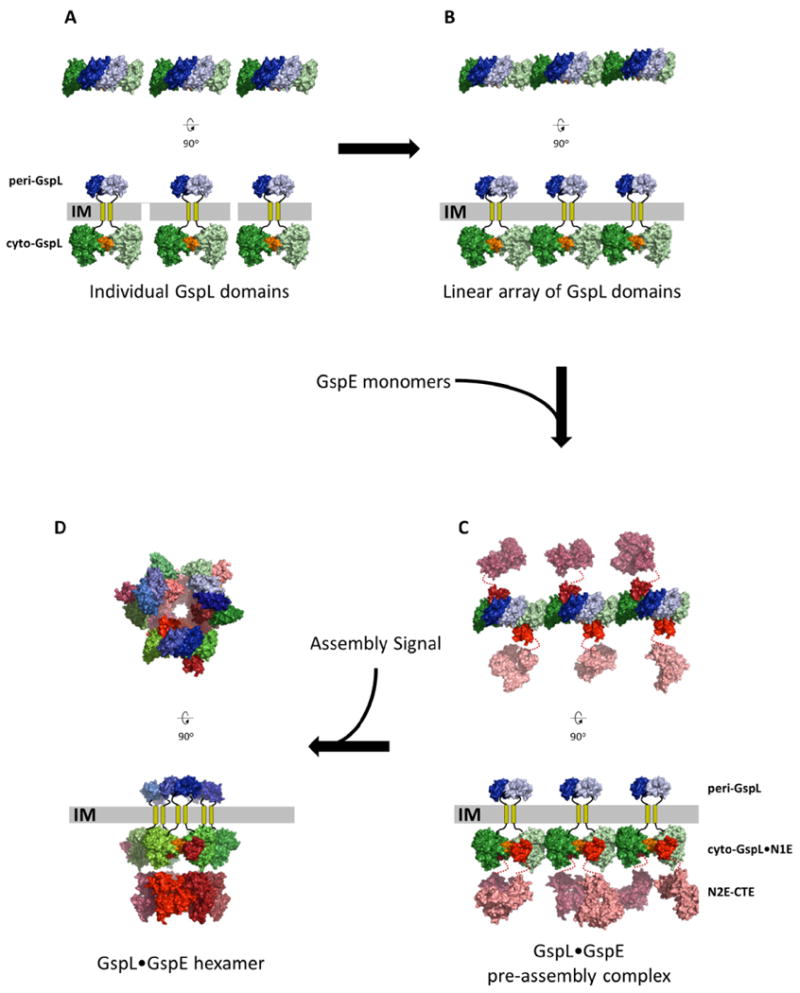
The upper views are perpendicular to the membrane. The lower views are parallel to the membrane.
A. Thre separate dimers of GspL with interactions across the α-interface (orange) of the cytoplasmic domains (green colors) and, e p-interface in the periplasmic domains (blue colors).
B. Multiple GspL dimers form linear arrays by β-interfaces amongst cytoplasmic domains.
C. N1E domains of GspE interact with cyto-GspL domains of a linear array, yielding a pre-assembly complex. Additional proteins like GspM (not shown) might also be part of the pre-assembly complex (see text).
D. After an assembly signal, six GspL and six GspE subunits form an assembly with the lower N2E-CTE didomain as a hexamer with approximate C6 symmetry, connected by N1E-N2E linker residues to the N1E•GspL complex with approximate C3 symmetry. The latter C3 axis relates three N1E•GspL dimers with each of these dimers containing an approximate C2 axis. The aforementioned approximate C6, C3 and C2 axes run parallel to each other, perpendicular to the inner membrane plane, with the C6 and C3 axes coinciding. The C2 axes have a different position, approximately related by the C3 axis.
