Figure 4.
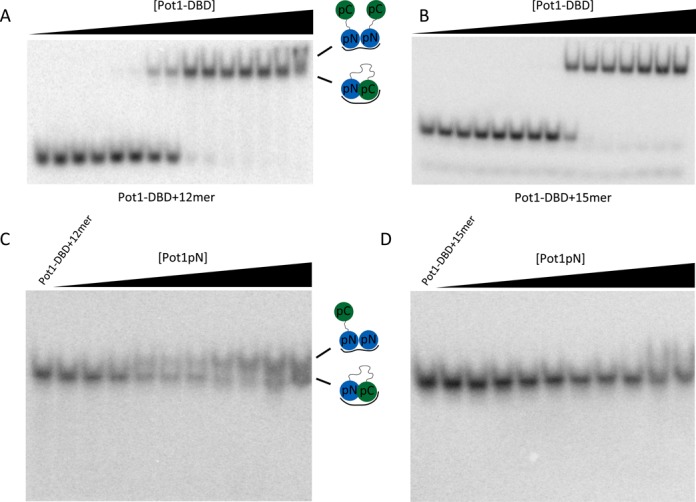
The 3′ end of 12mer, but not 15mer, is available for protein interaction when bound by Pot1-DBD. (A) Pot1-DBD begins to form a larger complex visible by EMSA in the presence of 12mer (presumably the 2:1 complex observed by SEC-MALS in Figure 3). Presumed complexes present are shown in cartoon. (B) In the presence of 15mer, however, there is no evidence of a larger, supershifted species at high concentrations of Pot1-DBD. Concentrations of Pot1-DBD are 0.34 fM, 3.4 fM, 34 fM, 68 fM, 340 fM, 680 fM, 3.4 pM, 6.8 pM, 34 pM, 68 pM, 340 pM, 680 pM, 6.8 nM, 68 nM and 680 nM. (C) Titration of Pot1pN into a solution of Pot1-DBD+12mer also forms a larger Pot1-DBD+Pot1pN+12mer complex. (D) Pot1pN, however, fails to form a larger complex with Pot1-DBD+15mer. Concentrations of Pot1pN are 0 pM, 200 pM, 400 pM, 2 nM, 4 nM, 20 nM, 40 nM, 200 nM, 400 nM, 2 μM and 4 μM.
