Figure 2.
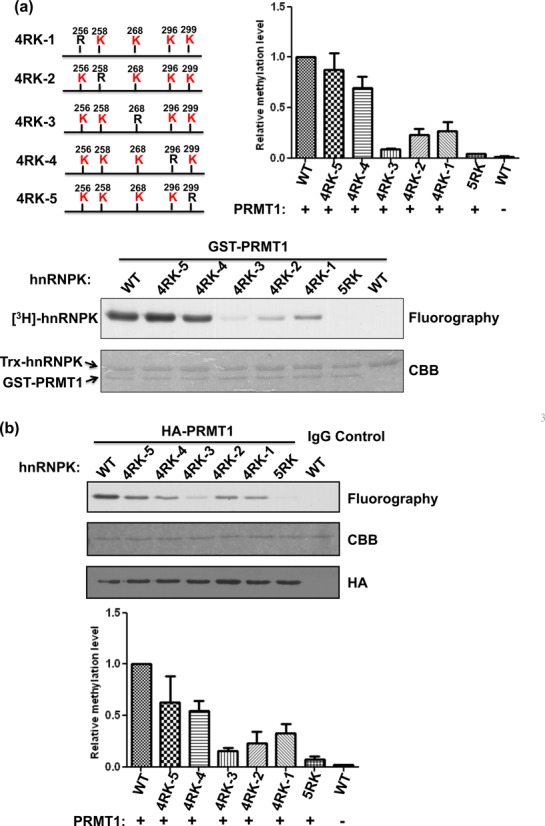
PRMT1 preferentially methylates Arg296 and Arg299 of hnRNPK. (a) Detection and quantification of the recombinant PRMT1-mediated methylation of WT hnRNPK, the 5RK mutant and five 4RK mutants, containing only one remaining arginine for methylation (as shown in the schematic diagram). HnRNPKs were incubated with GST-PRMT1 in the presence of [3H] S-adenosylmethionine (SAM), followed by SDS-PAGE. The proteins were stained with Coomassie blue (bottom). Methylation was detected through fluorography (top) and quantified using a liquid scintillation counter. The relative methylation levels of all mutants to WT hnRNPK are shown as a bar graph. (b) Detection and quantification of the PRMT1 complex-mediated methylation of WT hnRNPK, the 5RK mutant and five 4RK mutants in vitro. HA-PRMT1 complex were immunoprecipitated from the HEK293 cells using an anti-HA antibody and incubated with diverse hnRNPKs in the presence of [3H] S-adenosylmethionine (SAM). After SDS-PAGE analysis, the proteins were stained with Coomassie blue (bottom). Methylation was detected through fluorography (top) and quantified using a liquid scintillation counter. The relative methylation levels of all mutants compared with WT hnRNPK are shown as a bar graph. CBB, Coomassie Brilliant Blue.
