Figure 6.
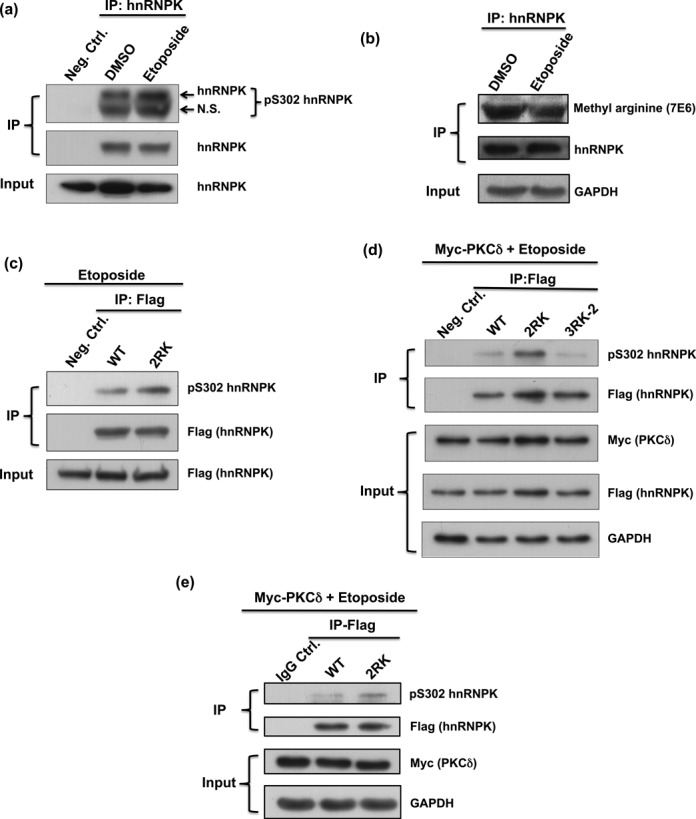
Loss of Arg296 and Arg299 methylation on hnRNPK promotes the nearby Ser302 phosphorylation under etoposide treatment in vivo. (a) Ser302 phosphorylation of endogenous hnRNPK was up-regulated upon etoposide treatment. U2OS cells were treated with or without etoposide for 4 h. Endogenous hnRNPKs were immunoprecipitated from U2OS lysates, followed by western blot analysis to reveal the Ser302 phosphorylation of hnRNPK. N.S., non-specific. (b) Etoposide treatment reduced methylation level of endogenous hnRNPK. The U2OS cells were treated with or without etoposide, and followed by immunoprecipitation of hnRNPK. Subsequent methylation detection and expression of the precipitated hnRNPKs were carried out using pan-asymmetric methyl-arginine antibody and hnRNPK antibody, respectively. (c) Arg296 and Arg299 mutations increased the Ser302 phosphorylation of hnRNPK in U2OS cells treated with etoposide. U2OS cells were transfected with Flag-WT or Flag-2RK (R296K/R299K) hnRNPK, followed by treatment with etoposide for 4 h. The exogenous hnRNPKs were immunoprecipitated from U2OS lysates using a Flag antibody followed by western blot analysis to determine the hnRNPK Ser302 phosphorylation status. (d) hnRNPK methylation at Arg296 and Arg299, but not the other arginines, affects Ser302 phosphorylation. U2OS cells carrying overexpressed Myc-tag PKCδ were transfected with Flag-WT or Flag-2RK (R296K/R299K) or Flag-3RK-2 (R256K/R258K/R268K) hnRNPK, followed by etoposide treatment for 4 h. The exogenous hnRNPKs were immunoprecipitated from U2OS lysates using a Flag antibody followed by western blot analysis to determine the hnRNPK Ser302 phosphorylation state. (e) Mutations at Arg296 and Arg299 increased hnRNPK Ser302 phosphorylation in H1299 cells treated with etoposide. H1299 cells carrying overexpressed Myc-tag PKCδ were transfected with Flag-WT or Flag-2RK (R296K/R299K) hnRNPK, followed by treatment with etoposide for 4 h. The exogenous hnRNPKs were immunoprecipitated from H1299 lysates, followed by western blot analysis to reveal the hnRNPK Ser302 phosphorylation status.
