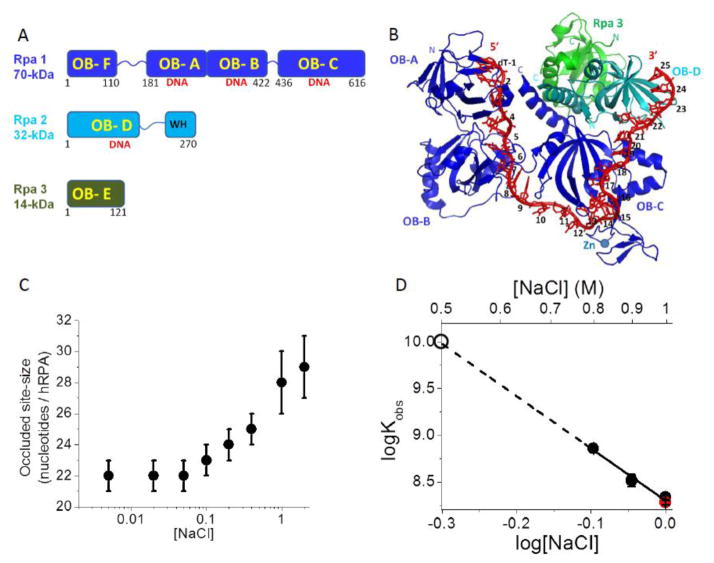
Figure 1. Binding of hRPA to ssDNA.
(A) Subunit composition of the hetero-trimeric hRPA showing the six OB-folds. (B) Structure of Ustilago maydis RPA bound to (dT)32 showing OB-folds A, B, and C of Rpa1 and OB-fold D of Rpa2 interacting with 25 nucleotides (PDB ID 4GOP)[11]. (C) The occluded site-size of hRPA bound to poly(dT) is dependent on [NaCl] (Buffer T, 25.0°C). (D) Dependence on [NaCl] of the equilibrium association constant (Kobs) for hRPA binding to (dT)30 (log-log plot) (Buffer T, 25.0°C). The dashed line shows a linear extrapolation to obtain an estimate of Kobs = 1x1010 M−1 at 0.50 M NaCl (open circle). A measurement of Kobs for hRPA binding to (dT)29-Cy3-T-3′ (red circle) is also shown at 1.0 M NaCl indicating that the presence of Cy3 on the DNA has little effect on Kobs.