Abstract
Purpose of review
The aim of this communication is to provide an up-to-date overview of myofibrillar myopathies (MFMs).
Recent findings
The most important recent advance in the MFMs has been the identification of mutation Bag3 (Bcl-2-associated athanogene-3) as a new cause of MFM. Although, the typical clinical manifestations of MFMs are slowly progressive weakness, the patients with Bag3opathy may have had a rapidly progressive and more severe phenotype.
Summary
Several MFM disease genes have recently been recognized. The identified disease proteins (desmin, αB-crystallin, myotilin, Zasp, filamin C, and Bag3) interact with components or with chaperones of the Z-disk. In each case the molecular defect leads to a largely stereotyped cascade of structural perturbation of the muscle fiber architecture.
Keywords: Myofibrillar myopathy, desmin, αB-crystallin, myotilin, Zasp, filamin C, Bag3, FHL1
Introduction
The myofibrillar myopathies (MFMs) are a group of muscular dystrophies associated with common morphologic features [1,2]. These consist of myofibrillar disorganization commencing at the Z-disk followed by accumulation of myofibrillar degradation products and ectopic expression of multiple proteins that include desmin, dystrophin, myotilin, sarcoglycans, neural cell adhesion molecule (NCAM), plectin, gelsolin, ubiquitin, filamin C, Xin, TAR DNA-binding protein 43 (TDP-43), and co-chaperones including αB-crystallin (Fig. 1C), heat shock protein (Hsp) 27 and DNAJB2, and congophilic amyloid material [3–10]. The diagnosis of MFM is established by muscle biopsy. The pathologic changes are best illustrated in trichrome stained sections of diseased muscle (Fig. 1A). The abnormal fibers harbor an admixture of amorphous, granular, or hyaline deposits that vary in shape and size, and are dark blue or blue red in color. Many abnormal fiber regions, and especially the hyaline structures, are devoid of, or have diminished, oxidative enzyme activity. Some hyaline structures are intensely congophilic. Some muscle fibers harbor small to large vacuoles containing membranous material and in some cases, some fibers are highly enlarged (giant fibers). The abnormal fibers can be distributed focally so if only a small muscle specimen is obtained, or if a clinically unaffected muscle is sampled, or if only paraffin embedded tissue is examined, the characteristic changes could be overlooked.
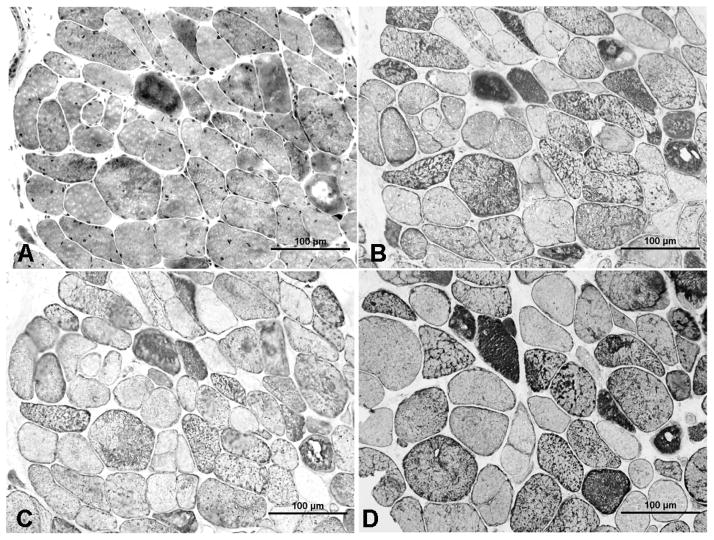
Figure 1.
Sections from nonconsecutive sections from a patient with Bag3opathy. Sections (A), (B) and (C) are from the same series. Many abnormal fibers in trichrome section (A) show abnormal accumulation of Bag3 (B), αB-crystallin (C) and heat shock protein 27 (D).
Electron microscopy shows that disintegration of the myofibrils begins at or in immediate proximity of the Z-disk. This is followed by accumulation of degraded filamentous material in various patterns, aggregation of membranous organelles and glycogen in spaces vacated by myofibrils, and degradation of dislocated membranous organelles in autophagic vacuoles.
Most patients with MFM present with progressive muscle weakness but in some patients, the cardiomyopathy may precede the muscle weakness. The disease is usually transmitted by autosomal dominant inheritance but infrequently X-linked or autosomal recessive inheritance is observed. Sporadic cases are frequent either because a mutation arises in the germ line, or because the disease in the parents was unrecognized. Peripheral neuropathy can be an associated feature. EMG studies of affected muscles reveal myopathic motor unit potentials and abnormal electrical irritability which often includes myotonic discharges.
Several MFM disease genes have been identified but in the Mayo MFM cohort of 80 patients, only 35 patients had a genetic diagnosis.
Desminopathy Subset of MFM
Since the first description of desminopathy by Goldfarb et al. [11] and Munoz-Marmol et al. [10], more than 40 mutations have been reported. The distribution of weakness can be distal, limb-girdle and scapuloperoneal. Muscle atrophy, mild facial weakness, dysphagia, dysarthria, and respiratory insufficiency can occur. Cardiomyopathy, especially arrhythmogenic type, is a common manifestation. The majority of patients present between 10 to 61 years of age but a patient with syncopal episodes since infancy carries a homozygous in-frame deletion of 7 amino acids (p.Arg173_Glu179del) in DES exon 6 [12]. In two brothers with childhood onset progressive axial and proximal muscle weakness, calf hypertrophy, severe joint contractures and dilated cardiomyopathy resembling “Emery-Dreifuss muscular dystrophy” phenotype, the disease was caused by a homozygous deletion of 22 bp in DES exon 6. Interestingly, the muscle biopsy does not show the typical MFM changes but a detailed pathologic description is not yet reported [13].
In a recent expression study, de novo aggregation properties of three GFP tagged-desmin mutants were investigated using confocal single-particle fluorescence spectroscopy. The Arg350Pro and Glu413Lys mutants form aggregates in HEK (human embryonal kidney) 293 cells that lack endogenous desmin, and Arg454Trp-mutant forms a filamentous network like wild-type desmin. Spectroscopy shows that the Arg350Pro mutant inhibits desmin assembly, the Glu413Lys mutant forms hyperstable tetramers, and the Arg454Trp mutant shows only a subtle defect in filament assembly [14]. However, another expression system using MEF Vim −/− (Vimentin knockout mouse embryo fibroblast) cells shows the Arg454Trp mutant forms abnormally short filamentous structures and only forms a filamentous network when cotransfected with wild-type desmin [15]. This paradox highlights that in vitro models may not yield clear insight into the pathogenic effects of the mutants in vivo, and that care needs to be exercised in the use and interpretation of expression studies.
αB-Crystallinopathy Subset of MFM
In 1998, Vicart et al. [16] identified a heterozygous missense mutation in CRYAB in a large kinship. Subsequently, two heterozygous truncating mutations were observed in this gene in 2 patients in the Mayo MFM cohort [17]. The affected patients presented in adult life, had symmetric proximal and distal muscle weakness and atrophy as well as respiratory involvement. Some patients also had hypertrophic cardiomyopathy, palato-pharyngeal weakness, and cataracts [16]. In 2010, another patient with CRYAB mutation (Gly145Ser) was reported [18]. The patient presented at age 68 years with slowly progressive distal leg weakness and intermittent atrial fibrillation. The muscle biopsy was typical of MFM.
Myotilinopathy Subset of MFM
In 2000, Hauser et al. [19] detected a missense mutation in MYOT in a large kinship that had previously been linked to the myotilin locus at 5q31. The disease was identified as limb-girdle muscular dystrophy 1A (LGMD1A). Two years later, a second kinship with a similar phenotype was found to have a missense mutation in myotilin [20]. Because myotilin is a Z-disk component, we searched for myotilin mutations in the Mayo cohort of MFM patients and identified six mutations in 8 unrelated patients [3]. Therefore, the morphologic substrate of LGMD1A is MFM pathology. Moreover, we found that distal myopathy, cardiomyopathy and peripheral neuropathy can be facets of myotilinopathy. Subsequent studies by other investigators identified additional patients with mutations in MYOT, including the kinship originally described under the rubric of “spheroid body myopathy” [6,21–23]. Some kinships also show intrafamily phenotypic variability. The majority of the MYOT mutations are heterozygous missense amino acid changes in exon 2 [3,6,19–22] but recently an Arg405Lys mutation was discovered in exon 9, in the second immunoglobulin-like domain of myotilin which is important for homodimer formation and protein-protein interaction [24*]. Expression studies demonstrated decreased homodimerization and decreased binding of myotilin to α-actinin, the backbone of the Z-disk.
Zaspopathy Subset of MFM
Zaspopathy was first described in 2005 by Selcen and Engel [5] in 11 MFM patients who carried heterozygous missense mutations (Ala147Thr and Ala165Val) in ZASP. The mean age of onset was in 6th decade. The patients had proximal and/or distal muscle weakness. Three patients had cardiac involvement without signs of coronary artery disease and in one patient the cardiac symptoms antedated the muscle weakness by 10 years. Peripheral nerve involvement by clinical, EMG, or histologic criteria was detected in 5 patients. Subsequently, a large kinship, originally described by Markesbery et al. [25], and 5 other kinships with distal myopathy and MFM pathology were shown to carry the Ala165Val in ZASP [26].
Recent knockdown experiments of the Drosophila ortholog of human ZASP demonstrated the importance of Zasp in muscle structure. Knockdown flies cannot fly and do not form Z-disks or recruit α-actinin to the Z-disk [27,28].
Filaminopathy Subset of MFM
In 2005, Vorgerd et al. [7] identified a dominant Trp2710X mutation in the last exon of FLNC in a large German kinship. Then, two additional German families were reported with the same nonsense mutation in filamin C. These families may have a common founder [29]. Subsequently, 3 MFM kinships carrying the same mutation were identified in the Mayo MFM cohort. The age of onset is between 24 and 60 years. All patients have progressive muscle weakness. The serum CK level is normal to 10-fold elevated above the upper limit of normal. Cardiomyopathy, respiratory insufficiency and peripheral neuropathy are associated features.
Recently, two other kinships were reported to have an in frame deletion (Val903_Thr933del) [30] and a complex deletion-insertion mutation (Lys899_Val904del and Val899_Cys900ins) [31] in the seventh Ig-like repeat of FLNC at exon 18. Interestingly, three patients in a large Chinese family had chronic diarrhea before the onset of muscle weakness [31].
Bag3opathy Subset of MFM
Bag3 is a multidomain co-chaperone protein interacting with many other polypeptides. Like other members of the Bag family, it harbors a C-terminal BAG domain that mediates interaction with Hsp 70, antiapoptotic protein Bcl-2, and a proline-rich region that interacts with WW-domain proteins implicated in signal transduction and with Src-3 homology (SH3)-domain proteins such as phospholipase Cγ-1 that also participates in antiapoptotic pathways. Bag3 itself has a unique N-terminal WW domain that binds proline-rich sequences. Bag3 forms a stable complex with the small Hsp 8 and participates in the degradation of misfolded or aggregated proteins [32–34]. Bag3 is strongly expressed in skeletal and cardiac muscle and at a lower level in other tissue s. Its targeted deletion in mice results in a fulminant myopathy with early lethality [35].
In 2009, Selcen et al. [9**] described Bag3opathy in 3 MFM patients who were heterozygous for Pro209Leu in exon 3. All three presented in childhood with severe progressive muscle weakness. All had cardiomyopathy and developed respiratory insufficiency with diaphragm paralysis by the second decade. Two also had a rigid spine. The muscle weakness was only proximal in one patient, both proximal and distal in the second patient, and distal more than proximal in the third patient. The serum CK ranged from 3 to 15 times above the upper limit of normal. EMG of one patient showed both axonal and demyelinating polyneuropathy. The light microscopy findings were typical of MFM (Fig. 1). The 3 patients differed from most other MFM patients in early age of onset, rapid evolution of the illness, and presence of the rigid spine. Apoptosis was found in 8% of the nuclei. The enhanced nuclear apoptosis in Bag3opathy is consistent with known antiapoptotic effect of Bag3 [33,36,37] and indicates that Pro209 contributes to this effect.
FHL1opathy
A wide clinical and pathologic spectrum is associated with FHL1 mutations. This includes late onset X-linked scapulo-axio-peroneal myopathy with bent spine syndrome (XMPMA) [38], reducing body myopathy (RBM) [39,40*], X-linked dominant scapuloperoneal myopathy [41], rigid spine syndrome [42] and Emery-Dreifuss muscular dystrophy like phenotype [43,44]. Although some pathologic features of the FHL1opathies and the MFMs overlap, reducing bodies have not been observed in other forms of MFMs [40*,45*].
Conclusion
MFMs are muscular dystrophies with distinguishing but not always identical morphologic features. The phenotypes include limb-girdle muscular dystrophy, distal myopathy, scapuloperoneal syndrome or rigid spine syndrome. The responsible disease genes in the majority of MFM kinships await discovery.
Acknowledgments
Work in the author’s laboratory was supported by a K08 NS050106 grant from NIH and Mayo Foundation CR20 Award.
References and recommended reading
- 1.Nakano S, Engel AG, Waclawik AJ, Emslie-Smith AM, Busis NA. Myofibrillar myopathy with abnormal foci of desmin positivity. I. Light and electron microscopy analysis of 10 cases. J Neuropathol Exp Neurol. 1996;55:549–562. doi: 10.1097/00005072-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 2.De Bleecker JL, Engel AG, Ertl BB. Myofibrillar myopathy with abnormal foci of desmin positivity. II. Immunocytochemical analysis reveals accumulation of multiple other proteins. J Neuropathol Exp Neurol. 1996;55:563–577. doi: 10.1097/00005072-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–1371. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
- 4.Selcen D, Ohno K, Engel AG. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain. 2004;127:439–451. doi: 10.1093/brain/awh052. [DOI] [PubMed] [Google Scholar]
- 5.Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 2005;57:269–276. doi: 10.1002/ana.20376. [DOI] [PubMed] [Google Scholar]
- 6.Olive M, Goldfarb LG, Shatunov A, Fischer D, Ferrer I. Myotilinopathy: refining the clinical and myopathological phenotype. Brain. 2005;128:2315–2326. doi: 10.1093/brain/awh576. [DOI] [PubMed] [Google Scholar]
- 7.Vorgerd M, van der Ven PF, Bruchertseifer V, Lowe T, Kley RA, Schroder R, Lochmuller H, Himmel M, Koehler K, Furst DO, et al. A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet. 2005;77:297–304. doi: 10.1086/431959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olive M, Janue A, Moreno D, Gamez J, Torrejon-Escribano B, Ferrer I. TAR DNA-Binding protein 43 accumulation in protein aggregate myopathies. J Neuropathol Exp Neurol. 2009;68:262–273. doi: 10.1097/NEN.0b013e3181996d8f. [DOI] [PubMed] [Google Scholar]
- 9**.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65:83–89. doi: 10.1002/ana.21553. This paper identifies for the first time mutations in BAG3 as a cause of MFM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claeys KG, Sozanska M, Martin JJ, Lacene E, Vignaud L, Stockholm D, Laforet P, Eymard B, Kichler A, Scherman D, et al. DNAJB2 Expression in Normal and Diseased Human and Mouse Skeletal Muscle. Am J Pathol. 2010;176:2901–2910. doi: 10.2353/ajpath.2010.090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfarb LG, Park KY, Cervenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino-Mora C, Sivakumar K, Dalakas MC. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet. 1998;19:402–403. doi: 10.1038/1300. [DOI] [PubMed] [Google Scholar]
- 12.Pinol-Ripoll G, Shatunov A, Cabello A, Larrode P, de la Puerta I, Pelegrin J, Ramos FJ, Olive M, Goldfarb LG. Severe infantile-onset cardiomyopathy associated with a homozygous deletion in desmin. Neuromuscul Disord. 2009;19:418–422. doi: 10.1016/j.nmd.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmignac V, Sharma S, Arbogast S, Fischer D, Serreri C, Serria M, Stoltenburg G, Maurage CA, Herrmann H, Cuisset JM, et al. A homozygous desmin deletion causes an Emery-Dreifuss like recessive myopathy with desmin depletion. Neuromuscular Disorders. 2009;19:600–600. [Google Scholar]
- 14.Levin J, Bulst S, Thirion C, Schmidt F, Botzel K, Krause S, Pertl C, Kretzschmar H, Walter MC, Giese A, et al. Divergent molecular effects of desmin mutations on protein assembly in myofibrillar myopathy. J Neuropathol Exp Neurol. 2010;69:415–424. doi: 10.1097/NEN.0b013e3181d71305. [DOI] [PubMed] [Google Scholar]
- 15.Bar H, Schopferer M, Sharma S, Hochstein B, Mucke N, Herrmann H, Willenbacher N. Mutations in desmin’s carboxy-terminal “tail” domain severely modify filament and network mechanics. J Mol Biol. 2010;397:1188–1198. doi: 10.1016/j.jmb.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 17.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54:804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 18.Reilich P, Schoser B, Schramm N, Krause S, Schessl J, Kress W, Muller-Hocker J, Walter MC, Lochmuller H. The p.G154S mutation of the alpha-B crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord. 2010;20:255–259. doi: 10.1016/j.nmd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Hauser MA, Horrigan SK, Salmikangas P, Torian UM, Viles KD, Dancel R, Tim RW, Taivainen A, Bartoloni L, Gilchrist JM, et al. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum Mol Genet. 2000;9:2141–2147. doi: 10.1093/hmg/9.14.2141. [DOI] [PubMed] [Google Scholar]
- 20.Hauser MA, Conde CB, Kowaljow V, Zeppa G, Taratuto AL, Torian UM, Vance J, Pericak-Vance MA, Speer MC, Rosa AL. Myotilin mutation found in second pedigree with LGMD1A. Am J Hum Genet. 2002;71:1428–1432. doi: 10.1086/344532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penisson-Besnier I, Talvinen K, Dumez C, Vihola A, Dubas F, Fardeau M, Hackman P, Carpen O, Udd B. Myotilinopathy in a family with late onset myopathy. Neuromuscul Disord. 2006;16:427–431. doi: 10.1016/j.nmd.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Foroud T, Pankratz N, Batchman AP, Pauciulo MW, Vidal R, Miravalle L, Goebel HH, Cushman LJ, Azzarelli B, Horak H, et al. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–1940. doi: 10.1212/01.wnl.0000188872.28149.9a. [DOI] [PubMed] [Google Scholar]
- 23.Berciano J, Gallardo E, Dominguez-Perles R, Garcia A, Garcia-Barredo R, Combarros O, Infante J, Illa I. Autosomal dominant distal myopathy with a myotilin S55F mutation: sorting out the phenotype. J Neurol Neurosurg Psychiatry. 2007;79:205–208. doi: 10.1136/jnnp.2007.125435. [DOI] [PubMed] [Google Scholar]
- 24*.Shalaby S, Mitsuhashi H, Matsuda C, Minami N, Noguchi S, Nonaka I, Nishino I, Hayashi YK. Defective myotilin homodimerization caused by a novel mutation in MYOT exon 9 in the first Japanese limb girdle muscular dystrophy 1A patient. J Neuropathol Exp Neurol. 2009;68:701–707. doi: 10.1097/NEN.0b013e3181a7f703. This paper demonstrates the decreased homodimerization and decreased binding of mutant myotilin to α-actinin. [DOI] [PubMed] [Google Scholar]
- 25.Markesbery WR, Griggs RC, Leach RP, Lapham LW. Late onset hereditary distal myopathy. Neurology. 1974;24:127–134. doi: 10.1212/wnl.24.2.127. [DOI] [PubMed] [Google Scholar]
- 26.Griggs R, Vihola A, Hackman P, Talvinen K, Haravuori H, Faulkner G, Eymard B, Richard I, Selcen D, Engel A, et al. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007;130:1477–1484. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- 27.Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179:1583–1597. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benna C, Peron S, Rizzo G, Faulkner G, Megighian A, Perini G, Tognon G, Valle G, Reggiani C, Costa R, et al. Post-transcriptional silencing of the Drosophila homolog of human ZASP: a molecular and functional analysis. Cell Tissue Res. 2009;337:463–476. doi: 10.1007/s00441-009-0813-y. [DOI] [PubMed] [Google Scholar]
- 29.Kley RA, Hellenbroich Y, van der Ven PFM, Furst DO, Huebner A, Bruchertseifer V, Peters SA, Heyer CM, Kirschner J, Schroder R, et al. Clinical and morphological phenotype of the filamin myopathy: a study of 31 German patients. Brain. 2007;130:3250–3264. doi: 10.1093/brain/awm271. [DOI] [PubMed] [Google Scholar]
- 30.Shatunov A, Olive M, Odgerel Z, Stadelmann-Nessler C, Irlbacher K, van Landeghem F, Bayarsaikhan M, Lee HS, Goudeau B, Chinnery PF, et al. In-frame deletion in the seventh immunoglobulin-like repeat of filamin C in a family with myofibrillar myopathy. Eur J Hum Genet. 2009;17:656–663. doi: 10.1038/ejhg.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luan X, Hong D, Zhang W, Wang Z, Yuan Y. A novel heterozygous deletion-insertion mutation (2695–2712 del/GTTTGT ins) in exon 18 of the filamin C gene causes filaminopathy in a large Chinese family. Neuromuscul Disord. 2010;20:390–396. doi: 10.1016/j.nmd.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 33.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’?--A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32. doi: 10.1016/s0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 34.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008;283:1437–1444. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 35.Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169:761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Buchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503:151–157. doi: 10.1016/s0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- 37.Bonelli P, Petrella A, Rosati A, Romano MF, Lerose R, Pagliuca MG, Amelio T, Festa M, Martire G, Venuta S, et al. BAG3 protein regulates stress-induced apoptosis in normal and neoplastic leukocytes. Leukemia. 2004;18:358–360. doi: 10.1038/sj.leu.2403219. [DOI] [PubMed] [Google Scholar]
- 38.Windpassinger C, Schoser B, Straub V, Hochmeister S, Noor A, Lohberger B, Farra N, Petek E, Schwarzbraun T, Ofner L, et al. An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. Am J Hum Genet. 2008;82:88–99. doi: 10.1016/j.ajhg.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schessl J, Zou Y, McGrath MJ, Cowling BS, Maiti B, Chin SS, Sewry C, Battini R, Hu Y, Cottle DL, et al. Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. J Clin Invest. 2008;118:904–912. doi: 10.1172/JCI34450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Schessl J, Taratuto AL, Sewry C, Battini R, Chin SS, Maiti B, Dubrovsky AL, Erro MG, Espada G, Robertella M, et al. Clinical, histological and genetic characterization of reducing body myopathy caused by mutations in FHL1. Brain. 2009;132:452–464. doi: 10.1093/brain/awn325. This is a comprehensive review of a large cohort of FHL1opathy patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinzii CM, Vu TH, Min KC, Tanji K, Barral S, Grewal RP, Kattah A, Camano P, Otaegui D, Kunimatsu T, et al. X-linked dominant scapuloperoneal myopathy is due to a mutation in the gene encoding four-and-a-half-LIM protein 1. Am J Hum Genet. 2008;82:208–213. doi: 10.1016/j.ajhg.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalaby S, Hayashi YK, Goto K, Ogawa M, Nonaka I, Noguchi S, Nishino I. Rigid spine syndrome caused by a novel mutation in four-and-a-half LIM domain 1 gene (FHL1) Neuromuscul Disord. 2008;18:959–961. doi: 10.1016/j.nmd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Gueneau L, Bertrand AT, Jais JP, Salih MA, Stojkovic T, Wehnert M, Hoeltzenbein M, Spuler S, Saitoh S, Verschueren A, et al. Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2009;85:338–353. doi: 10.1016/j.ajhg.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knoblauch H, Geier C, Adams S, Budde B, Rudolph A, Zacharias U, Schulz-Menger J, Spuler A, Yaou RB, Nurnberg P, et al. Contractures and hypertrophic cardiomyopathy in a novel FHL1 mutation. Ann Neurol. 2010;67:136–140. doi: 10.1002/ana.21839. [DOI] [PubMed] [Google Scholar]
- 45*.Schoser B, Goebel HH, Janisch I, Quasthoff S, Rother J, Bergmann M, Muller-Felber W, Windpassinger C. Consequences of mutations within the C terminus of the FHL1 gene. Neurology. 2009;73:543–551. doi: 10.1212/WNL.0b013e3181b2a4b3. This is a comprehensive overview of the consequences of C-terminal FHL1 mutations. [DOI] [PubMed] [Google Scholar]