Abstract
A short and efficient total synthesis of the alkaloid isosolenopsin and its enantiomer has been achieved. The key step was a ω-transaminase-catalysed regioselective monoamination of the diketone pentadecane-2,6-dione, which was obtained in a single step through the application of a Grignard reaction. Initial low conversions in the biotransformation could be overcome by optimisation of the reaction conditions employing suitable cosolvents. In the presence of 20 vol.-% N,N-dimethylformamide (DMF) or n-heptane the best results were obtained by employing two enantiocomplementary ω-transaminases originating from Arthrobacter at 30–40 °C; under these conditions, conversions of more than 99 % and perfect stereocontrol (ee > 99 %) were achieved. Diastereoselective chemical reduction (H2/Pd/C) of the biocatalytic product gave the target compound. The linear three-step synthesis provided the natural product isosolenopsin in diastereomerically pure form (ee > 99 %, dr = 99:1) with an overall yield of 64 %.
Keywords: Piperidine, Alkaloids, Amination, Enzymes, Diastereoselectivity
Introduction
Functionalised chiral piperidines are popular key elements in a vast number of synthetic protocols and are among the most common skeletal fragments found in natural products.1 Due to the wide range of useful pharmacological properties, extensive efforts have been devoted to the (stereoselective) synthesis of such compounds.2 In particular, simple 2,6-disubstituted piperidines constitute an important class of natural products due to their biological activity and abundant occurrence in nature. Prominent examples are the secreted fire ant venom alkaloids from Solenopsis invitica; selected members of this family were found to display cytotoxic, insecticidal, hemolytic, antibacterial, antifungal and necrotic properties.3 Furthermore, physiological investigations revealed that for example, isosolenopsin A (1b) selectively inhibits designated neuronal nitric oxide synthases (nNOS),4 whereas solenopsin A (2b) inhibits the regulator protein phosphatidylinositol-3-kinase (PI3K); the latter is involved in controlling apoptosis, proliferation and angiogenesis.5
These diverse biological activities and the apparent simple structure of piperidines (Figure 1) have made attractive targets to study and showcase new synthetic methods. Several racemic and asymmetric total syntheses have been reported based on chiral pool precursors and catalytic (asymmetric) transformations, in addition to electrochemical and auxiliary controlled methods.6 Although several routes are very elegant and involve highly sophisticated key steps, the long reaction sequences and the use of an orthogonal protecting-group strategy has hampered their overall efficiency. Hence, more capable and environmental begin methods are still desired.
Figure 1.
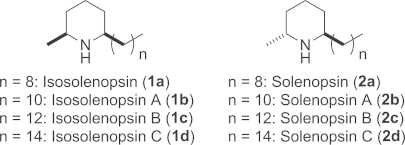
Secreted fire ant venom alkaloids and their structure.
Recently, we demonstrated that 2,6-diketones 3 serve as useful precursors for the synthesis of the optically pure 2,6-disubstituted piperidine scaffold 8 through enzymatic reductive amination (Scheme 1).7 A ω-transaminase (ω-TA)8–10 catalysed regio- and stereoselective monoamination of 3 at the sterically less demanding (ω-1)-keto moiety, yielding preferentially amino-ketone 4, which underwent spontaneous ring-closure to give the corresponding Δ1-piperideines 6 in enantiomerically pure form. Based on this concept, we now report an extension of this method for the total synthesis of isosolenopsin [(2S,6R)-1a] and its enantiomer (2R,6S)-1a.11
Scheme 1.
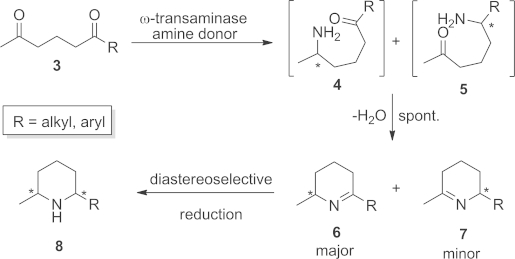
Chemoenzymatic asymmetric synthesis of 2,6-disubstituted piperidines involving regio- and stereoselective monoamination.
Results and Discussion
Various enantiocomplementary ω-TAs were tested for the transformation of diketone 3a into the corresponding Δ1-piperideines 6a and 7a under designated reaction conditions by employing alanine as amine donor (Scheme 2). The equilibrium was shifted towards the product side by removal/recycling of the formed pyruvate with an alanine-dehydrogenase (AlaDH).12 Unfortunately, only low conversions were achieved, probably due to the low solubility of diketone 3a in pure buffer solution. Subsequently, various water-miscible organic solvents [acetonitrile, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), 1,2-dimethoxyethane (DME), dimethyl sulfoxide (DMSO)] and immiscible solvents (EtOAc, toluene) were assayed at 10 vol.-%. Although most of the investigated ω-TAs gave insufficient conversions (less than 5 %), four showed conversions of around 25 %, rendering them targets for a more detailed study. Notably, regioisomer 7a was not detected for any enzyme investigated (see the Supporting Information).
Scheme 2.
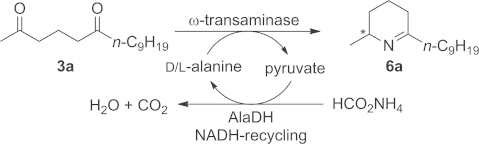
Monoamination of diketone 3a to yield Δ1-piperideine 6a. Reagents and conditions: lyophilised E. coli cells containing the overexpressed ω-TA (20 mg), diketone 3a (12 mg, 50 mm), PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), D- or L-alanine (500 mm), AlaDH (12 U), FDH (11 U), organic cosolvent and KPi buffer (100 mm, pH 7.0), 30 °C, 24 h in an Eppendorf orbital shaker (700 rpm).
Focussing on the four most promising ω-TAs identified, originating from Arthrobacter citreus,13c Arthrobacter sp.13d Pseudomonas fluorescens,13a,13b and Hyphomonas neptunium,9f the influence of the concentration of water-miscible (DMF, DME, DMSO) and immiscible (toluene) solvents at concentrations in the range 5–40 vol.-% was investigated [for the analytical scale: 25 mm 3a (6 mg/mL); 1.00 mL total volume]. The first two enzymes are (S)-selective, whereas the latter two are (R)-selective. The conversion obtained depended on the solvent and on the enzyme employed (Figure 2); whereas the use of enzymes from P. fluorescens and H. neptunium led to conversions of 25 % at maximum, the two enantiocomplementary ω-TAs from Arthrobacter turned out to be superior. For example, the (S)-selective ω-TA from A. citreus gave conversions up to 60 % when DMF was applied. Notably, increasing the concentration of DMF led to higher conversions, whereas the reverse trend was observed for toluene or DME as cosolvent. In the case of toluene, this is probably due to the lower availability of the organic substrate in the aqueous phase. An analogous observation was made for the ω-TA from (R)-Arthrobacter sp. For this enzyme, piperidine 6a was obtained with 60 % conversion by applying 5 vol.-% toluene and 80 % conversion in the presence of 40 vol.-% DMF.
Having identified two suitable enzymes with opposite stereopreference [Arthrobacter citreus for the (S)- and Arthrobacter sp. for the (R)-enantiomer] and cosolvents, further optimisation of temperature and pH was conducted. The biocatalytic monoamination of 3a was tested at different pH values (ranging from 6.5 to 9.9) at varied DMF concentrations (20 and 40 vol.-%) (Figure 3). Subsequently, the conversion was studied as a function of temperature at varied DMF concentrations (20 and 40 vol.-%; Figure 4). Thereby, the highest conversion was obtained for the asymmetric reductive amination of diketone 3a at 20 vol.-% DMF, pH 6.5, and 30 °C with the ω-TA originating from (R)-Arthrobacter sp., whereas 20 vol.-% DMF (pH 7.0) and elevated temperatures of 40 °C were best suited for the (S)-selective ω-TA of Arthrobacter citreus.
Figure 4.
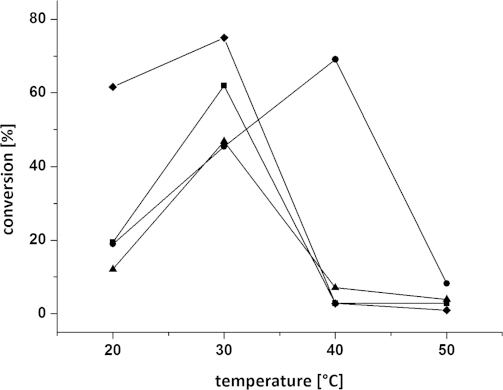
Influence of temperature on the conversion of diketone 3a at varying DMF concentrations: (♦) 40 vol.-% DMF, (R)-Arthrobacter sp., (▪) 20 vol.-% DMF, (R)-Arthrobacter sp., (•) 20 vol.-% DMF, A. citreus, (▴) 40 vol.-% DMF, A. citreus. Reagents and conditions: diketone 3a (25 mm), lyophilised E. coli cells containing the overexpressed ω-TA (20 mg), PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), D- or L-alanine (500 mm), AlaDH (12 U), FDH (11 U), 20–40 vol.-% DMF, KPi buffer (100 mm, pH 7.0), 20–50 °C, 24 h, 700 rpm.
Although conversions above 95 % were achieved by employing the reaction conditions mentioned above, n-heptane was also investigated due to the similarity in polarity of the solvent to the starting material 3a (Figure 5). For solubility reasons, the substrate still needed to be predissolved in DMF (5 vol.-% final conc.). Despite the formation of a biphasic system, the reaction resulted in full conversion (> 99 %) at 20 vol.-% n-heptane for the ω-TA from (R)-Arthrobacter sp. and 85 % conversion for the (S)-ω-TA from Arthrobacter.
Figure 5.
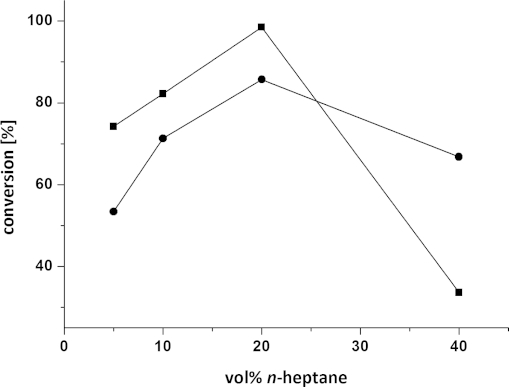
Reductive amination of diketone 3a in an aqueous two-phase system: (▪) (R)-Arthrobacter sp., (•) A. citreus. Reagents and conditions: diketone 3a (25 mm) dissolved in 5 vol.-% DMF, lyophilised E. coli cells containing the overexpressed ω-TA (20 mg), PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), D- or L-alanine (500 mm), AlaDH (12 U), FDH (11 U), 5–40 vol.-% n-heptane, KPi buffer (100 mm, pH 7.0), 30 °C, 24 h, 700 rpm.
The synthetic potential was finally demonstrated in the total synthesis of both enantiomers of isosolenopsin [(2S,6R)-1a and (2R,6S)-1a]. Starting with a Grignard reaction, the required diketone 3a was obtained in a single step from commercially available dihydropyrane-2-one 9 in 65 % yield (Scheme 3). The subsequent biotransformation was conducted under the optimised conditions on an increased scale (36 mg, 0.15 mmol, 25 mm); the ω-TA of Arthrobacter sp. gave access to the (R)-enantiomer, whereas the ω-TA of Arthrobacter citreus gave the (S)-enantiomer of piperidine 6a. Both reactions yielded the corresponding cyclic imine (R)- and (S)-6a, respectively, in optically pure form (ee > 99 %) at full conversion (> 99 % in both cases). Notably, the reaction times were prolonged (48 h) to ensure full conversion and hence simplify the work up procedures. Due to the instability of imine 6a, it was directly subjected to diastereoselective reduction using hydrogen with Pd/C as catalyst after extraction. Under these conditions, the second stereocentre was established under perfect substrate control, affording the natural product (2S,6R)-1a and (2S,6R)-1a in diastereomerically pure form [dr (syn/anti) = 99 % as deduced by GC and NMR analysis] in an excellent yield of 94–98 % (over two steps). Thus, starting from pyranone 9, the natural alkaloids (+)-1a and (–)-1a were obtained in optically pure form in 64 % overall yield.
Scheme 3.
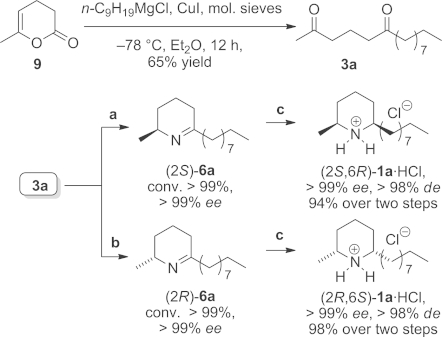
Chemoenzymatic synthesis of both enantiomers of isosolenopsins (2S,6R)-1a and (2R,6S)-1a under the optimised reaction conditions. Reagents and conditions: (a) Diketone 3a (36 mg, 0.15 mmol, 25 mm) dissolved in DMF (20 vol.-%), ω-TA from Arthrobacter citreus, PLP (1 mm), NAD+ (1 mm), L-alanine (20 equiv.), ammonium formate (150 mm), 11 U FDH, 12 U AlaDH, 48 h, 40 °C, 700 rpm; (b) same as for (a) but with the ω-TA from (R)-Arthrobacter sp. at 30 °C with D-alanine and 20 vol.-% n-heptane, 5 vol.-% DMF; (c) Pd/C, H2 (1 atm.), 4 h, 22 °C; precipitation with etherial HCl solution (5 equiv.).
Conclusions
An efficient chemoenzymatic total synthesis of the alkaloid isosolenopsin and its enantiomer has been achieved by using a regioselective monoamination of the corresponding diketone in the key step. Optimisation of the biocatalytic reaction conditions through medium engineering allowed initial low conversions to be overcome. Among various organic solvents tested, n-heptane and DMF turned out to be best suited at 30 °C for the (R)-selective ω-TA from Arthrobacter sp. and at 40 °C for the (S)-selective ω-TA from Arthrobacter citreus. The final asymmetric reductive monoamination of diketone 3a proceeded with perfect regio- and stereoselectivity at full conversions. This strategy afforded the natural product in optically pure form with an overall yield of 64–65 % over three steps.
Experimental Section
General: All starting materials were obtained from commercial suppliers and used as received unless stated otherwise. Petroleum ether (PE), where used, had a boiling range 63–69 °C. The reactions were carried out with standard Schlenk techniques under an N2 atmosphere in oven-dried (120 °C) glassware. Preparative chromatographic separations were performed by column chromatography on Merck silica gel 60 (0.063–0.200 mm). TLC was carried out with precoated aluminium sheets (TLC Silica gel 60 F254, Merck) with detection by staining with cerium molybdenum solution or visualisation by UV. Optical rotation was measured at 20 °C with a Perkin–Elmer Polarimeter 341. GC–MS spectra were recorded with an Agilent 7890A GC-system, equipped with an Agilent 5975C mass-selective detector and a HP-5 MS column (30 m × 0.25 mm × 0.25 μm; hydrogen as carrier gas [flow = 0.55 mL/min]). 1H and 13C NMR spectra were recorded at 20 °C with a 300 Bruker NMR unit spectrometer; chemical shifts are given in ppm relative to Me4Si (1H: Me4Si = 0 ppm) or relative to the resonance of the solvent (1H: CDCl3 = 7.26 ppm; 13C: CDCl3 = 77.0 ppm). Formate dehydrogenase from Candida boidinii (2.2 Umg–1) [one unit will oxidize 1.0 μmol of formate to CO2 per min at pH 7.6 at 37 °C] was purchased from Codexis (catalogue no. FDH 002). Lyophilised E. coli cells containing overexpressed ω-TA were prepared as previously reported.9a,9b,9g Purified recombinant L-alanine dehydrogenase was prepared as described in the literature (15 μL of the crude solution are equal to 12 U).9b
Pentadecane-2,6-dione (3b): CuI (340 mg, 1.78 mmol) was added to a stirred solution of n-C9H19MgBr (1 m in Et2O, 8.82 mL, 8.82 mmol)] in Et2O (30 mL) and MS 3 Å (50 mg) in one portion. The mixture was stirred for 30 min at 21 °C and then cooled to –78 °C. Dihydro-2H-pyran-2-one 9 (1.00 g, 8.92 mmol) was added dropwise by using a syringe over a period of 15 min. The reaction mixture was kept at –78 °C, then Grignard reagent (0.5 equiv., 4.46 mL, 4.46 mmol) was added after 4 h and a further portion of Grignard reagent (0.5 equiv., 4.46 mL, 4.46 mmol) was added after 8 h. When TLC monitoring revealed full conversion of the starting material (10 h), the mixture was treated with aqueous HCl solution (1 n, 30 mL) and allowed to warm to room temperature. The reaction mixture was extracted with small portions of EtOAc (4×) and the organic layer was dried with MgSO4. Subsequent filter flash chromatography (PE/EtOAc, 90:10) of the concentrated solution afforded the diketone in 65 % yield (1.39 g, 5.79 mmol) as a colourless powder. Spectroscopic data are in agreement with those previously published.14 M.p 65–66 °C. Rf = 0.49 (PE/EtOAc, 80:20). 1H NMR (300 MHz, CDCl3): δ = 0.84 (t, 3J15,14 = 6.7 Hz, 3 H, 15-H), 1.22 (br. s, 12 H, 9-H, 10-H, 11-H, 12-H, 13-H and 14-H), 1.50 (tt, 3J8,7 = 7.3, 3J8,9 = 6.6 Hz, 2 H, 8-H), 1.80 (tt, 3J4,3 = 7.2, 3J4,5 = 7.1 Hz, 2 H, 4-H), 2.09 (s, 3 H, 1-H), 2.30 (t, 3J7,8 = 7.5 Hz, 2 H, 7-H), 2.37 (t, 3J3,4 = 7.2 Hz, 2 H, 3-H), 2.40 (t, 3J5,4 = 7.2 Hz, 2 H, 5-H) ppm. 13C NMR (75 MHz, CDCl3): δ = 14.2 (C-15), 17.8 (C-4), 22.7 (C-14), 23.9 (C-8), 29.3 (C-11), 29.3 (C-12), 29.5 (C-10), 29.5 (C-9), 30.0 (C-1), 31.9 (C-13), 41.5 (C-3), 42.6 (C-5), 42.9 (C-7), 208.5 (C-2), 210.9 (C-6) ppm. GC–MS (EI, 70 eV): m/z (%) = 240 (1) [M+], 155 (15) [C10H19O+], 128 (82) [C7H12O2+], 85 (65) [C5H9O+], 43 (100) [C2H3O+].
Optimisation of Reaction Conditions (Analytical Scale): Lyophilised cells of E. coli containing the corresponding overexpressed ω-TA (20 mg) were rehydrated in potassium phosphate buffer (100 mm at varying pH as indicated in Figures2, 3, 4, and 5) containing PLP (1.0 mm), NAD+ (1.0 mm), ammonium formate (150 mm), FDH (11 U), Ala-DH (12 U) and D- or L-alanine (500 mm) at room temperature for 30 min. Substrate 3a (6 mg, 25 mm, dissolved in the corresponding organic solvent as indicated in Figures2, 3, 4, and 5) was then added and the reductive amination was performed at defined temperature as indicated (Figures2, 3, 4, and 5) in an Eppendorf orbital shaker (700 rpm, vertical position) for 24 h. After the period of time, the reaction was stopped by addition of saturated Na2CO3 (200 μL) and vigorous shaking. The mixture was extracted with EtOAc (3 × 500 μL) and the combined organic layers were dried with MgSO4 and an aliquot was withdrawn for further analysis. Conversions were determined by GC analysis on an achiral phase [column HP-5 (Agilent); carrier gas: hydrogen; temperature program: 100 to 250 °C at 10 °C min–1]: Rt = 8.7 (6a), 10.6 (3a) min. Determination of enantiomeric excess by GC analysis on a chiral phase [column DEX-CB (Agilent; CP-Chirasil); carrier gas: helium; temperature program: 60 °C (1 min), then 6090 °C at 5.0 °C min–1 then 90180 °C at 2 °C min–1]: Rt = 37.23 [(2R)-6a], 37.41 [(2S)-6a] min.
Figure 2.
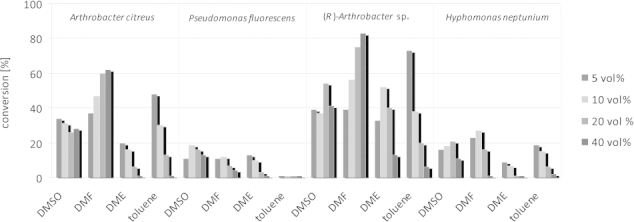
Conversions for the monoamination of diketone 3a in the presence of various organic cosolvents employing (R)- and (S)-selective ω-TAs. Reagents and conditions: diketone 3a (6 mg/mL, 25 mm), lyophilised E. coli cells containing overexpressed ω-TA (20 mg), PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), D- or L-alanine (500 mm), AlaDH (12 U), FDH (11 U), organic cosolvent, K-phosphate buffer (100 mm, pH 7.0), 30 °C, 24 h in an Eppendorf orbital shaker (700 rpm).
Figure 3.
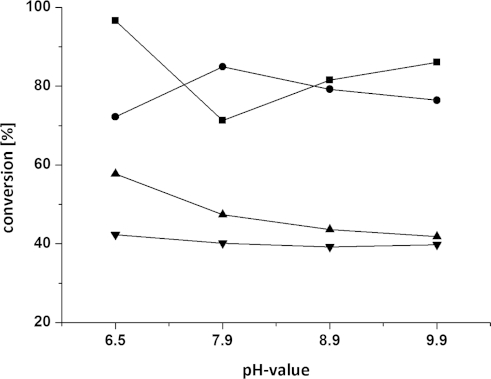
Influence of pH on the conversion of diketone 3a at varying DMF concentrations: (•) 20 vol.-% DMF, (R)-Arthrobacter sp., (▪) 40 vol.-% DMF, (R)-Arthrobacter sp., (▴) 20 vol.-% DMF, A. citreus, (▾) 40 vol.-% DMF, A. citreus. Reagents and conditions: diketone 3a (25 mm), lyophilised E. coli cells containing the overexpressed ω-TA (20 mg), PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), D- or L-alanine (500 mm), AlaDH (12 U), FDH (11 U), 20–40 vol.-% DMF, KPi buffer (100 mm), 30 °C, 24 h, 700 rpm.
Biocatalytic Synthesis of Δ1-Piperideine (R)-6a (Preparative Scale): Lyophilised cells of E. coli containing overexpressed ω-TA from (R)-Arthrobacter sp. (125 mg) were rehydrated in K-phosphate buffer (4.8 mL, pH 6.5, 100 mm) containing PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), FDH (11 U), Ala-DH (12 U) and D-alanine (500 mm) at 22 °C for 30 min. Substrate 3a [36 mg (dissolved in 300 μL DMF), 0.15 mmol, 25 mm], and n-heptane (1.2 mL) was added afterwards and the reaction was shaken for 48 h at 30 °C. Saturated aqueous Na2CO3 solution was added (1 mL) and the reaction mixture was extracted with EtOAc (4 × 5 mL). Combined organic layers were dried with Na2SO4, filtered and converted without further purification. The conversion and optical purity was determined as described above. GC–MS (EI+, 70 eV): m/z (%) = 223 (2) [M+], 124 (25) [C8H14N+], 111 (100) [C7H13N+], 96 (61) [C6H10N+].
Biocatalytic Synthesis of Δ1-Piperideine (S)-6a (Preparative Scale): Lyophilised cells of E. coli containing overexpressed ω-TA from Arthrobacter citreus (125 mg) were rehydrated in KPi buffer (5.7 mL, pH 6.5, 100 mm) containing PLP (1 mm), NAD+ (1 mm), ammonium formate (150 mm), FDH (11 U), Ala-DH (12 U) and L-alanine (500 mm) at 22 °C for 30 min. Substrate 3a [36 mg (dissolved in 1.2 mL of DMF), 0.15 mmol, 25 mm] was added and the reaction was shaken for 48 h at 40 °C. Saturated Na2CO3 solution was added (0.50 mL) and the reaction mixture was extracted with EtOAc (4 × 5 mL). The combined organic layers were dried with Na2SO4, filtered and converted without further purification. The conversion and optical purity was determined as described above.
Diastereoselective Reduction; Typical Synthesis of (–)-Isosolenopsin [(2S,6R)-1a]: A crude solution of the biotransformation containing the corresponding cyclic imine (S)-6a was treated with palladium on activated charcoal (10 wt.-%). The mixture was stirred vigorously and a stream of hydrogen was bubbled through the solution for 4 h. After full conversion of the starting material (monitored by GC and GC–MS analyses), the solution was filtered through a small plug of Celite 545 and cooled to 0 °C. Etherial HCl solution was added dropwise (ca. 5 equiv.), the solvent removed, and the precipitate was collected. If necessary, the product was recrystallised from pure CHCl3 or purified by filter flash chromatography (Et2O + 1 vol.-% 2-PrNH2). (+)-Isosolenopsin [(2S,6R)-1a] was obtained as a colourless solid in 98 % yield (38.5 mg, 0.15 mmol) over two steps. Analytical data are in full agreement with those previously published.6a M.p. 172 °C. RF = 0.35 (Et2O + 1 vol.-% 2-PrNH2). 1H NMR (300 MHz, CDCl3): δ = 0.85 (t, 3J14,13 = 6.9 Hz, 3 H, 14-H), 1.15–1.50 (m, 15 H), 1.55 (d, 3JMe,1 = 6.3 Hz, 3 H, Me), 1.55–2.05 (m, 6 H), 2.06–2.20 (m, 1 H), 2.88 (mc, 1 H, 1-H), 3.06 (mc, 1 H, 6-H), 9.01 (br. s, 1 H, NH2+), 9.39 (br. s, 1 H, NH2+) ppm. 13C NMR (75 MHz, CDCl3): δ = 14.2 (C-14), 19.6 (Me at C-1), 22.8, 23.0, 25.8, 27.6, 29.4, 29.5, 29.6, 29.7, 30.9, 32.0, 33.4, 54.7 (C-5), 58.8 (C-1) ppm. GC–MS (EI+, 70 eV): m/z (%) = 224 (1) [M+], 98 (100) [C6H12N+]. [α]D20 = –9.0 (c = 1.0, CHCl3, ee > 99 %, de > 98 %); Lit.:6a [α]D25 = +11.1 (c = 1.02, CHCl3) for the opposite enantiomer.
Synthesis of (+)-Isosolenopsin [(2R,6S)-1a]: The product was prepared as described for its enantiomer, starting with the crude solution obtained from the biotransformation with (R)-Arthrobacter sp. (see above). [α]D20 = +8.7 (c = 0.96, CHCl3, ee > 99 %, de > 98 %).
Supporting Information (see footnote on the first page of this article): Initial experiments (enzyme testings), and GC-MS plus NMR data of all products and intermediates.
Acknowledgments
This work was supported by the Austrian Ministry of Economy, Family and Youth (BMWFJ), the Austrian Ministry for Transport, Innovation and Technology (BMVIT), the Steirische Wirtschaftsförderung (SFG), the Standortagentur Tirol and the Technologieagentur der Stadt Wien (ZIT) through the Austria FFG-COMET-Funding Program. H. L. acknowledges DK “Molecular Enzymology” and financial support by the FWF (Project W9). Financial support by NAWI-Graz is acknowledged. The authors thank Barbara Grischek for her ongoing support in all projects.
Supporting Information
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/ejoc.201300157.
miscellaneous_information
References
- 1a.Bates RW, Sa-Ei K. Tetrahedron. 2002;58:5957–5978. [Google Scholar]
- 1b.Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. Tetrahedron: Asymmetry. 2000;11 [Google Scholar]
- 2a.Girling PR, Kiyoi T, Whitning A. Org. Biomol. Chem. 2011;9:3105–3121. doi: 10.1039/c0ob00996b. [DOI] [PubMed] [Google Scholar]
- 2b.Buffat MGP. Tetrahedron. 2004;60:1701–1729. [Google Scholar]
- 2c.Bailey PD, Millwood PA, Smith PD. Chem. Commun. 1983:633–640. [Google Scholar]
- 2d.Baliah V, Jeyaraman R, Chandrasekaran L. Chem. Rev. 1998;83:379–423. [Google Scholar]
- 3a.Breakman JC, Daloze D, Pasteels JM. Roberts MF, Wink M. Alkaloids: Biochemistry Ecology, and Medicinal Applications. Plenum Press: 1998. pp. 349–374. [Google Scholar]
- 3b.Breakman JC, Daloze D. J. Braz. Chem. Soc. 1996;7:251–256. [Google Scholar]
- 3c.Jones TH, Blum MS. Tetrahedron. 1982;38:1949–1958. [Google Scholar]
- 4.Yi GB, McClendon D, Desaiah D, Goddard J, Lister A, Moffitt J, Vander Meer RK, deShazo R, Lee KS, Rockhold RW. Int. J. Toxicol. 2003;22:81–86. doi: 10.1080/10915810305090. [DOI] [PubMed] [Google Scholar]
- 5.Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, Vlahos CJ, Ye K, Perry BN, Matter W, Fischl A, Cook J, Silver PA, Bain J, Cohen P, Whitmire D, Furness S, Govindarajan B, Bowen JP. Blood. 2007;109:560–565. doi: 10.1182/blood-2006-06-029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Gouault N, Roch ML, de Campos Pinto G, David M. Org. Biomol. Chem. 10:5541–5546. doi: 10.1039/c2ob25685a. For selected examples since 2005, see: [DOI] [PubMed] [Google Scholar]
- 6b.Reddy CR, Latha B. Tetrahedron: Asymmetry. 2011;22:1849–1854. [Google Scholar]
- 6c.Leijohndal K, Borén L, Braun R, Bäckvall J-E. J. Org. Chem. 2009;74:1988–1993. doi: 10.1021/jo8025109. [DOI] [PubMed] [Google Scholar]
- 6d.Kumar RSC, Sreedhar E, Reddy GV, Babu KS, Rao JM. Tetrahedron: Asymmetry. 2009;20:1160–1163. [Google Scholar]
- 6e.González-Gómez JC, Foubelo F, Yus M. Synlett. 2008:2777–2780. [Google Scholar]
- 6f.Takahata H, Saito Y, Ichnose M. Org. Biomol. Chem. 2006;4:1587–1595. doi: 10.1039/b601489e. [DOI] [PubMed] [Google Scholar]
- 6g.Dobbs AP, Guesné SJJ. Synlett. 2005:2101–2103. [Google Scholar]
- 6h.Wang X, Dong Y, Sun J, Xu X, Li R, Hu Y. J. Org. Chem. 2005;70:1897–1900. doi: 10.1021/jo0480444. [DOI] [PubMed] [Google Scholar]
- 6i.Girard N, Gautier C, Malassene R, Hurvois J-P, Moinet C, Toupet L. Synlett. 2004:2005–2009. [Google Scholar]
- 6j.Wang X, Dong Y, Sun J, Xu X, Li R, Hu Y. J. Org. Chem. 2005;70:1897–1900. doi: 10.1021/jo0480444. [DOI] [PubMed] [Google Scholar]
- 7a.Simon RC, Grischek B, Zepeck F, Steinreiber A, Belaj F, Kroutil W. Angew. Chem. 2012;124:6817. doi: 10.1002/anie.201202375. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2012;51:6713–6716. doi: 10.1002/anie.201202375. 6820. [DOI] [PubMed] [Google Scholar]
- 7b.Simon RC, Zepeck F, Kroutil W. Chem. Eur. J. 2013;19:2859–2865. doi: 10.1002/chem.201202793. [DOI] [PubMed] [Google Scholar]
- 8a.Mathew S, Yun H. ACS Catal. 2012;2:993–1001. For selected review articles, see: [Google Scholar]
- 8b.Malik MS, Park E-S, Shin J-S. Appl. Microbiol. Biotechnol. 2012;94:1163–1171. doi: 10.1007/s00253-012-4103-3. [DOI] [PubMed] [Google Scholar]
- 8c.Tufvesson P, Lima-Ramos J, Jensen JS, Al-Haque N, Neto W, Woodley JM. Biotechnol. Bioeng. 2011;108:1479–1493. doi: 10.1002/bit.23154. [DOI] [PubMed] [Google Scholar]
- 8d.Koszelewski D, Tauber K, Faber K, Kroutil W. Trends Biotechnol. 2010;28:324–332. doi: 10.1016/j.tibtech.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 8e.Turner NJ, Truppo M. Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Weinheim: Wiley-VCH; 2010. pp. 431–459. [Google Scholar]
- 8f.Hailes HC, Dalby PA, Lye GJ, Baganz F, Micheletti M, Szita N, Ward JM. Curr. Org. Chem. 2010;14:1883–1893. [Google Scholar]
- 8g.Ward J, Wohlgemuth R. Curr. Org. Chem. 2010;14:1914–1927. [Google Scholar]
- 9a.Steffen-Munsberg F, Vickers C, Thontowi A, Schätzle S, Tumlirsch T, Humble MS, Land H, Berglund P, Bornscheuer UT, Höhne M. ChemCatChem. 2013;5:150–153. For selected examples of ω-TA in asymmetric reductive amination, see: [Google Scholar]
- 9b.Mutti FG, Fuchs CS, Pressnitz D, Turrini NG, Sattler JH, Lerchner A, Skerra A, Kroutil W. Eur. J. Org. Chem. 2012:1003–1007. [Google Scholar]
- 9c.Mutti FG, Fuchs CS, Pressnitz D, Sattler JH, Kroutil W. Adv. Synth. Catal. 2011;353:3227–3233. [Google Scholar]
- 9d.Schätzle S, Steffen-Munsberg F, Thontowi A, Höhne M, Robins K, Bornscheuer U. Adv. Synth. Catal. 2011;353:2439–2445. [Google Scholar]
- 9e.Truppo MD, Rozzell JD, Turner NJ. Org. Process Res. Dev. 2010;14:234–237. [Google Scholar]
- 9f.Höhne M, Schätzle S, Jochens H, Robins K, Bornscheuer UT. Nat. Chem. Biol. 2010;6:807–813. doi: 10.1038/nchembio.447. [DOI] [PubMed] [Google Scholar]
- 9g.Fuchs M, Koszelewski D, Tauber K, Kroutil W, Faber K. Chem. Commun. 2010;46:5500–5502. doi: 10.1039/c0cc00585a. [DOI] [PubMed] [Google Scholar]
- 9h.Koszelewski D, Göritzer M, Clay D, Seisser B, Kroutil W. ChemCatChem. 2010;2:73–77. [Google Scholar]
- 9i.Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Javis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J, Devine PN, Huisman GW, Hughes GJ. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- 9j.Koszelewski D, Lavandera I, Clay D, Guebitz GM, Rozzell D, Kroutil W. Angew. Chem. 2008;120:9477–9482. doi: 10.1002/anie.200803763. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:9337–9340. doi: 10.1002/anie.200803763. [DOI] [PubMed] [Google Scholar]
- 9k.Koszelewski D, Lavandera I, Clay D, Rozzell D, Kroutil W. Adv. Synth. Catal. 2008;350:2761–2767. for selected examples of ω-TA in kinetic resolution, see: [Google Scholar]
- 9l.Cho B-K, Park H-Y, Seo J-H, Kim J, Kang T-J, Lee B-S, Kim B-G. Biotechnol. Bioeng. 2008;99:275–284. doi: 10.1002/bit.21591. [DOI] [PubMed] [Google Scholar]
- 9m.Bea H-S, Park H-J, Lee S-H, Yun H. Chem. Commun. 2008;47:5894–5896. doi: 10.1039/c1cc11528f. [DOI] [PubMed] [Google Scholar]
- 9n.Hanson RL, Davis BL, Goldberg SL, Johnston RM, Parker WL, Tully TP, Montana MA, Patel RN. Org. Process Res. Dev. 2008;12:1119–1129. [Google Scholar]
- 10a.Sehl T, Simon RC, Hailes HC, Ward JM, Schell U, Pohl M, Rother D. J. Biotechnol. 2012;159:188–194. doi: 10.1016/j.jbiotec.2011.12.023. For screening assays for ω-TA, see: [DOI] [PubMed] [Google Scholar]
- 10b.Hopwood J, Truppo MD, Turner NJ, Lloyed RC. Chem. Commun. 2011;47:773–775. doi: 10.1039/c0cc02919j. [DOI] [PubMed] [Google Scholar]
- 10c.Truppo MD, Rozzell JD, Moore JC, Turner NJ. Org. Biomol. Chem. 2009;7:395–398. doi: 10.1039/b817730a. [DOI] [PubMed] [Google Scholar]
- 11.Leclercq S, Thirionet I, Broeders F, Daloze D, Vander Meer R, Braekman JC. Tetrahedron. 1994;50:8465–8478. For the determination of the absolute configuration of cistrans- and -Solenopsins, see: [Google Scholar]
- 12a.Truppo MD, Turner NJ, Rozzell JD. Chem. Commun. 2009:2127–2129. doi: 10.1039/b902995h. For methods used to shift the equilibrium, see: [DOI] [PubMed] [Google Scholar]
- 12b.Höhne M, Kühl S, Robins K, Bornscheuer UT. ChemBioChem. 2008;9:363–365. doi: 10.1002/cbic.200700601. [DOI] [PubMed] [Google Scholar]
- 12c.Shin J-S, Kim B-G. Biotechnol. Bioeng. 1999;65:206–2011. [PubMed] [Google Scholar]
- 12d.Yun H, Yang Y-H, Cho B-K, Hwang B-Y, Kim B-G. Biotechnol. Lett. 2003;25:809–814. doi: 10.1023/a:1023500406897. [DOI] [PubMed] [Google Scholar]
- 12e.Cassimjee KE, Branneby C, Abedi V, Wells A, Berglund P. Chem. Commun. 2010;46:5569–5571. doi: 10.1039/c0cc00050g. [DOI] [PubMed] [Google Scholar]
- 13a.Kawano S, Ito N, Yasohara Y. 2007. WO 2007/139055.
- 13b.Ito N, Kawano S, Hagesawa J, Yasohara Y. Biosci. Biotechnol. Biochem. 2011;75:2093–2095. doi: 10.1271/bbb.110240. [DOI] [PubMed] [Google Scholar]
- 13c.Pannuri S, Kamat SV, Garcia ARM. (Cambrex North Brunswick Inc.) WO 2006/ 063336 A2. 2006 [Google Scholar]
- 13d.Yamada Y, Iwasaki A, Kizaki N. (Kaneka Corporation), EP 0987332 A1. 2000 [Google Scholar]
- 14.Abe K, Okumura H, Tsugoshi T, Nakamura N. Synlett. 1984:603–605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


