Scheme 3.
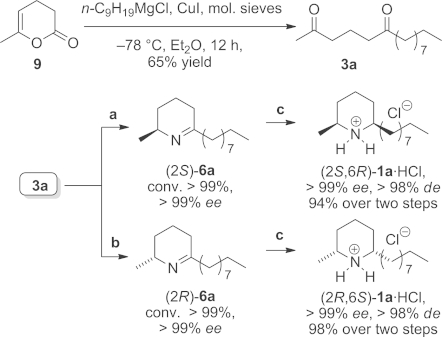
Chemoenzymatic synthesis of both enantiomers of isosolenopsins (2S,6R)-1a and (2R,6S)-1a under the optimised reaction conditions. Reagents and conditions: (a) Diketone 3a (36 mg, 0.15 mmol, 25 mm) dissolved in DMF (20 vol.-%), ω-TA from Arthrobacter citreus, PLP (1 mm), NAD+ (1 mm), L-alanine (20 equiv.), ammonium formate (150 mm), 11 U FDH, 12 U AlaDH, 48 h, 40 °C, 700 rpm; (b) same as for (a) but with the ω-TA from (R)-Arthrobacter sp. at 30 °C with D-alanine and 20 vol.-% n-heptane, 5 vol.-% DMF; (c) Pd/C, H2 (1 atm.), 4 h, 22 °C; precipitation with etherial HCl solution (5 equiv.).
