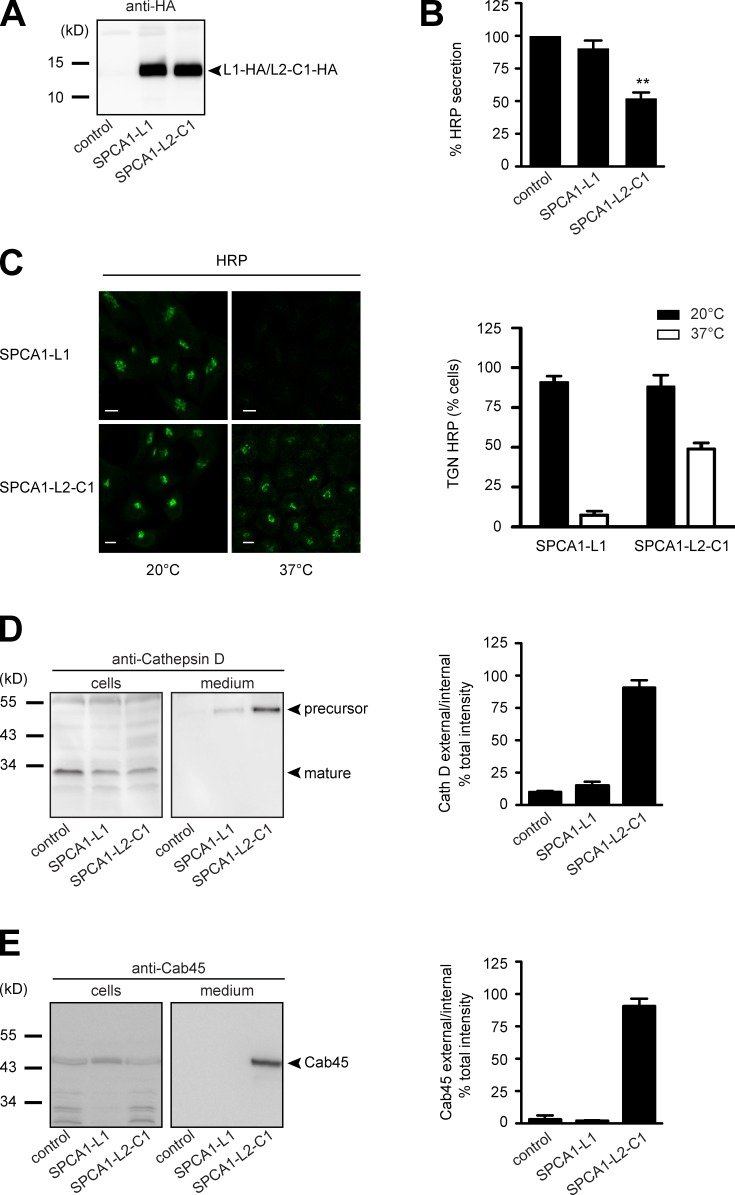
Figure 5.
The interaction of CFL-1 and actin to SPCA1-L2-C1 is crucial for protein sorting at the TGN. (A) HeLa cells expressing ss-HRP were transduced with a pLPCX plasmid (control), SPCA1-L1-HA, or SPCA1-L2-C1-HA. Cells were subsequently lysed and proteins were separated by SDS-PAGE. The presence of SPCA1-L1-HA and SPCA1-L2-C1-HA was determined by Western blotting with an anti-HA antibody. (B) Cell culture supernatants of cells described in A were analyzed for HRP activity by chemiluminescence. Error bars show mean ± SD of external HRP activity normalized to internal HRP activity of three independent experiments. Datasets were statistically significant when P < 0.01 (**). (C) HeLa cells stably expressing SPCA1-L1-HA or SPCA1-L2-C1-HA were incubated at 20°C for 2 h in the presence of cycloheximide to accumulate HRP in the TGN. Cells were subsequently shifted to 37°C, and the localization of HRP was analyzed by fluorescence microscopy with an anti-HRP antibody. To quantify the results, 100 cells expressing SPCA1-L1-HA or SPCA-L2-C1-HA in three different experiments at 20°C and 37°C were counted. Bars, 5 µm. (D and E) Media and lysates from the same cells were Western blotted with specific antibodies against Cathepsin D (D) or Cab45 (E). Western blots from three independent experiments were quantified by densitometry using the ImageJ software. Bar graphs represent the densitometry values of external Cathepsin D (D) and Cab45 (E) normalized to internal Cathepsin D and Cab45 values, respectively.