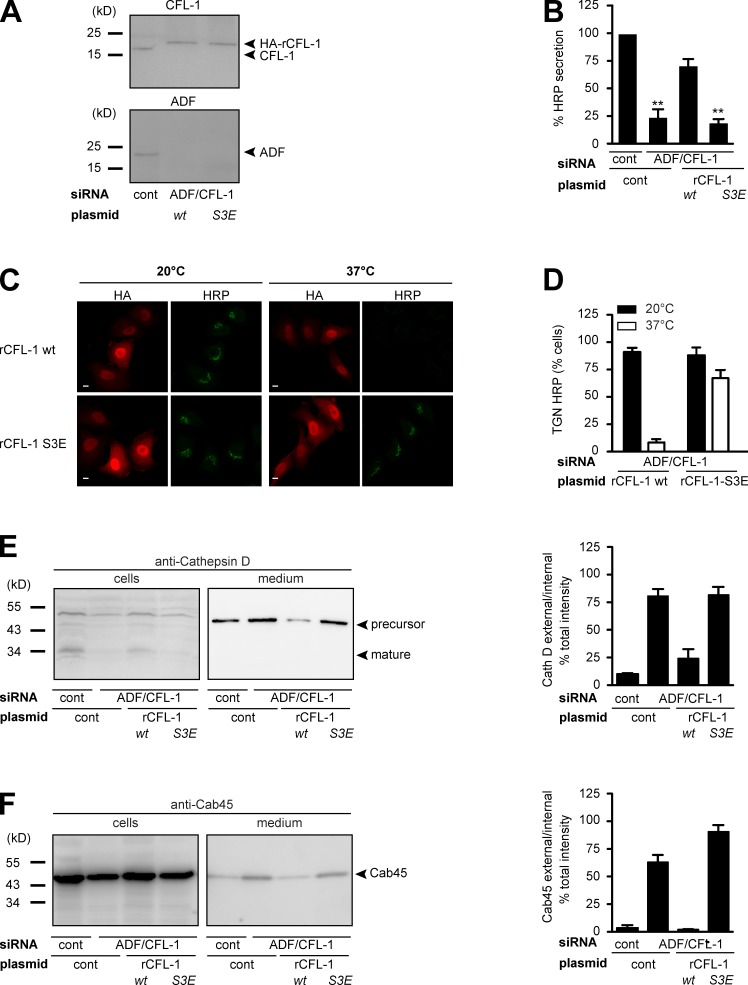
Figure 7.
Expression of CFL-1-S3E that does not bind to SPCA1 and actin causes missorting of secretory cargo. (A) HeLa cells were transfected with control or ADF and CFL-1 siRNA. In parallel, ADF and CFL-1 knockdown HeLa cells were transfected with siRNA-resistant rat HA-tagged CFL-1 (rCFL-1) wt or rCFL-1-S3E plasmids, and analyzed by SDS-PAGE and Western blotting with CFL-1 and ADF antibodies. (B) Cells described in A were transfected with ss-HRP-Flag. Medium from these cells was analyzed for HRP secretion. Error bars indicate the mean SD of HRP activity in the medium normalized by HRP activity in cell lysates collected from three independent experiments. (C) HeLa cells transfected with ADF/CFL-1 siRNA, ss-HRP-Flag, and HA-rCFL-1 wt or rCFL-1-S3E were incubated at 20°C for 2 h in the presence of cycloheximide to arrest HRP in the TGN. Cells were then shifted to 37°C, and the localization of HRP was analyzed with an HRP antibody in confocal microscopy. Bars, 5 µm. (D) 100 cells expressing ss-HRP-Flag and HA-rCFL-1 wt or rHA-CFL-1-S3E were counted in three different experiments at 20°C and 37°C. HeLa cells were transfected with control and ADF/CFL-1 siRNA and incubated for 40 h. Then ADF/CFL-1 knockdown cells were either transfected with a control plasmid or with rCFL-1 wt or rCFL-1-S3E. (E and F) Media and lysates from these cells were Western blotted with Cathepsin D (E) and Cab45 (F) antibodies. Western blots from three independent experiments were quantified by densitometry. Bar graphs represent the densitometry values of external Cathepsin D (E) and Cab45 (F) normalized to internal Cathepsin D and Cab45 values, respectively.