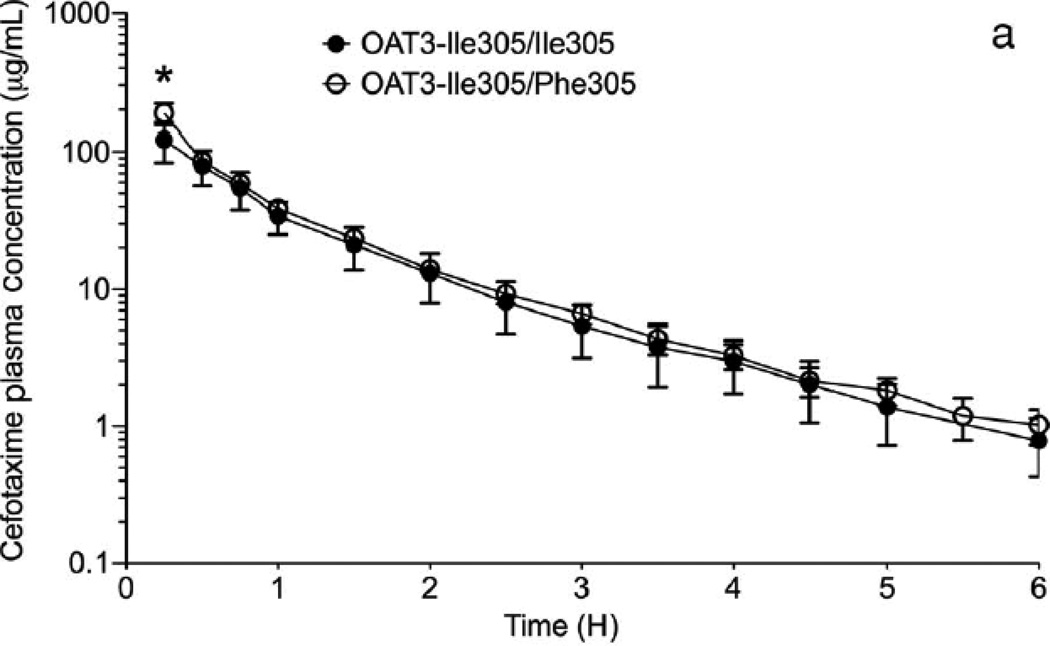
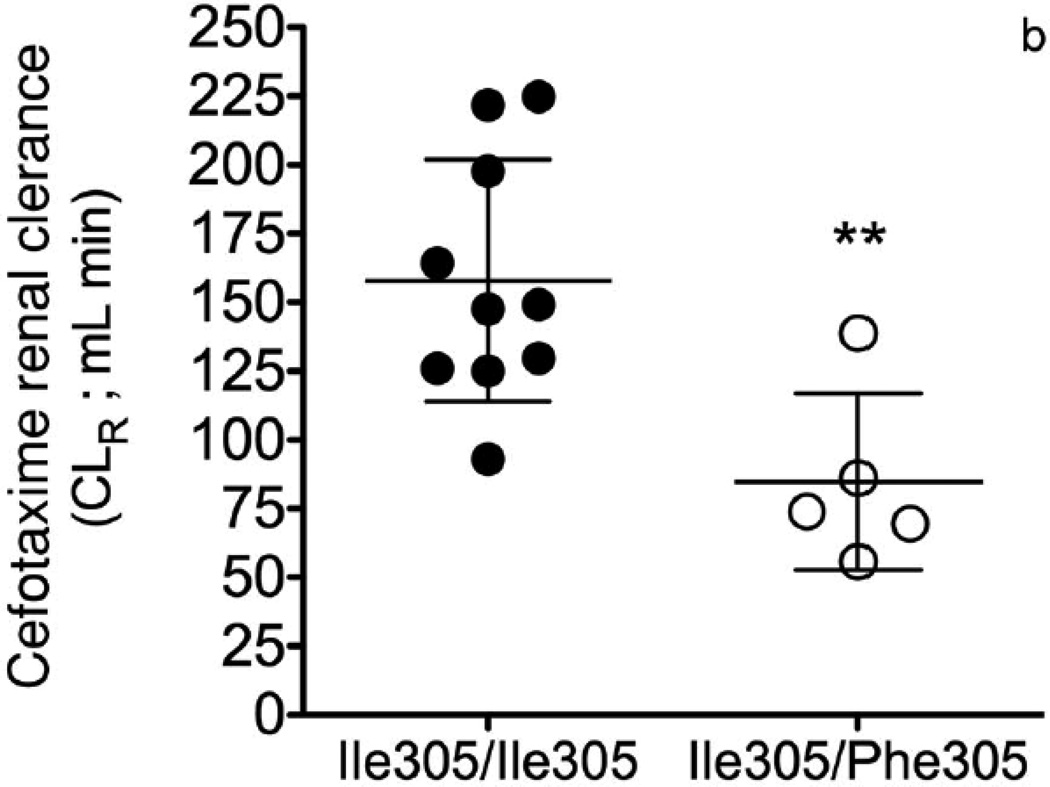
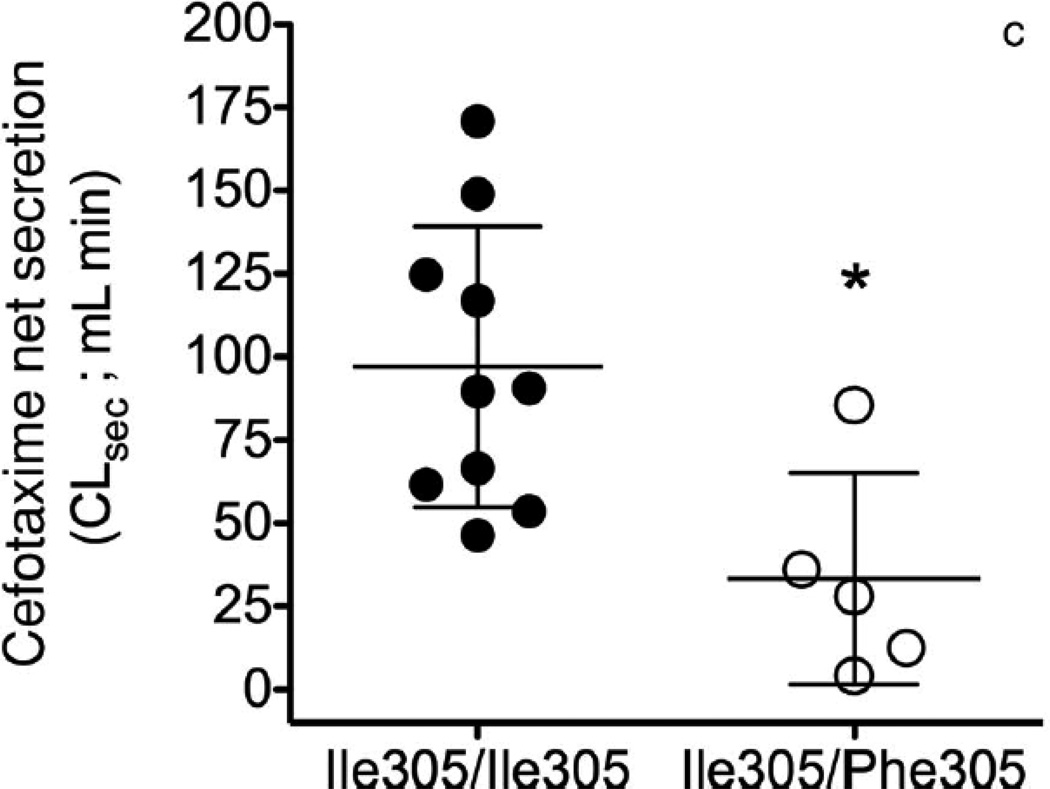
Figure 3.
Effect of OAT3 genotype on (a) plasma concentrations (b) renal clearance (CLR) and (c) net secretory clearance (CLsec) of cefotaxime in healthy volunteers. A single 2 gram dose of cefotaxime was administered as an intravenous bolus dose over 5 min to volunteers homozygous for the reference (Ile305/Ile305, n=10) or heterozygous for the variant (Ile305/Phe305, n=5). CLR parameters were determined using non-compartmental analyses. Data shown represent mean ± SEM (Figure 3(a)) or mean ± SD (Figure 3(b) and (c)). Significant differences between genotype groups as assessed by unpaired two-tailed t-test) are indicated as, * p<0.05, ** p<0.01 versus volunteers homozygous for the OAT3 reference allele. The pharmacokinetic parameters are shown in Table 1.