Abstract
Pigment epithelium-derived factor (PEDF) is a multifunctional factor with potent anti-angiogenic activity that may play a role in skin homeostasis and wound healing. Analysis of PEDF levels demonstrated that PEDF levels are high in normal skin but quite low in early wounds. As previous studies have suggested that keratinocytes can produce PEDF, we investigated how conditions that mimic those found at sites of injury influence PEDF production by keratinocytes in vitro. Both injury by mechanical disruption (scratch assay) and treatment of human keratinocytes with inflammatory cytokines (IL-1β, IL-6 and TNF-α) inhibited PEDF expression. We next examined how PEDF affects keratinocyte functions that are important in tissue repair. Treatment of keratinocytes with exogenous PEDF enhanced keratinocyte adhesion, therefore impairing migration, while having no effect on cell proliferation. The results suggest that modulation of PEDF levels may play a pivotal role in skin homeostasis and the response of keratinocytes to injury or inflammatory insults.
Keywords: pigment epithelium-derived factor, keratinocyte, wound, cytokine, in vitro
Background
PEDF (Serpinf1) is a 50 kDa secreted glycoprotein that belongs to the superfamily of serine protease inhibitors. PEDF has been demonstrated to be one of the most potent endogenously produced physiological and pathological anti-angiogenic factors (1, 2), and also has direct and indirect suppressive effect on tumor growth (3). PEDF has been detected in multiple tissues including brain, spinal cord, liver, bone, eye, heart, lung, and plasma (4). PEDF expression has also been reported in the dermal and epidermal layers of skin. Alterations in the levels of PEDF have been implicated in skin aging and the pathogenesis of some skin conditions, including melanoma, psoriasis and condyloma acuminatum (5-8). Given its functions and wide pattern of expression, PEDF has been proposed to be a factor that promotes the return to tissue homeostasis following pathologic insult (9). Evidence for such function has been described in the horse, a species in which PEDF levels have been shown to undergo modulation in skin wounds (10). During wound healing, keratinocytes proliferate and migrate across the wound bed, contributing to wound closure; these cells act upon and respond to signals from the underlying vascularized dermis. While it is likely that PEDF plays a role in the regulation of angiogenesis that is critical to skin homeostasis and recovery from injury, it is unknown whether secreted PEDF participates in the dynamic reciprocity between epidermal and dermal layers. Therefore, we examined the regulation of PEDF expression in keratinocytes under in vitro conditions that mimic those of a skin wound. The data demonstrates that PEDF expression in primary isolated skin keratinocytes is modulated by injury stimuli, including a mechanical disruption and inflammatory cytokines. Our results suggest a role for PEDF in modulating not only angiogenesis, but also keratinocyte function following tissue injury.
Questions addressed
Effects injury-related stimuli on PEDF expression in human skin keratinocytes in vitro; effects of PEDF on keratinocyte adhesion, migration, and proliferation in vitro.
Experimental design
Results
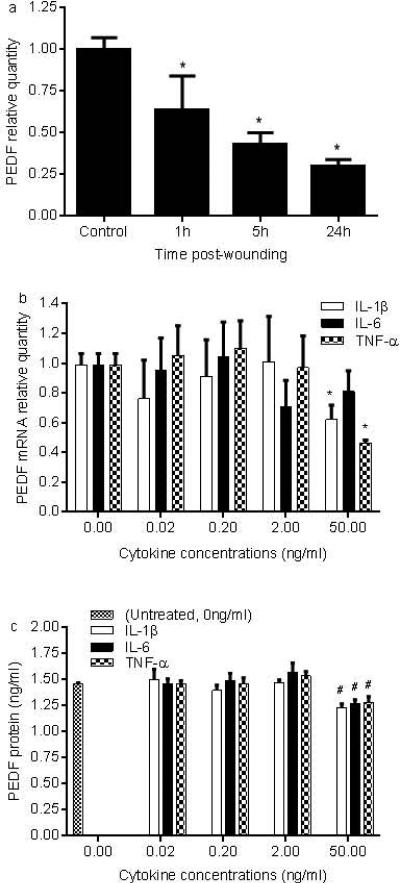
From our existing microarray database (11), the relative gene expression of PEDF in mouse skin wounds was significantly decreased at 12 hours post-wounding when compared to uninjured skin (8.9 vs 10.7, p<0.05). Resolving wounds (day 10) showed PEDF levels similar to control normal skin. In a mouse dermal burn wound model, PEDF was downregulated at 0 and 2 hours and upregulated at days 3 and 14 post-wounding (GEO profile ID2328534) (12), a pattern which was similar to our data. Comparable to early wounds, in vitro injury of NHEK by mechanical scratch wound also resulted in a significant decrease in PEDF mRNA expression from one to 24 hours after injury (Fig. 1a, p<0.01-0.001). The early wound environment contains multiple inflammatory cytokines; in particular IL-1β, IL-6, and TNF-αhave been shown to be markedly increased in early skin wounds or injured NHEK (11, 13). To examine if a proinflammatory environment similar to that found at sites of skin injury might influence PEDF expression, NHEK were exposed to a range of concentrations of IL-1β, IL-6, or TNF-α for 24 hours. Treatment with IL1β or TNF-α at the highest concentration of 50ng/ml resulted in a statistically significant decrease in PEDF mRNA expression (Fig. 1b, p<0.01). This result was further validated upon review of an available microarray GEO profile of IL-1β treated keratinocytes (ID 35957232) (14). IL-6 treatment also decreased the expression of PEDF at the two highest concentrations of 2 and 50ng/ml, although the decrease did not reach statistically significance (Fig.1b, p>0.05). Similar to the mRNA findings, levels of PEDF protein production were significantly decreased by each independent cytokine treatment at the 50ng/ml dose (Fig.1c, p<0.05).
Figure 1.
PEDF expression decreases in injured and inflammatory cytokine treated NHEK. a) NHEK monolayers were wounded by scratch injury. PEDF mRNA expression at 1, 5, and 24h after injury was analyzed by real time PCR. (*p<0.01-0.001 compared to uninjured control, t test). b,c) NHEK monolayers were treated with a range of concentrations of inflammatory cytokines (0.02-50ng/ml, IL-1β, IL-6 or TNF-α. PEDF mRNA and protein levels were examined 24h after treatment by real time PCR and ELISA respectively (*p<0.01, #p<0.05 compared to untreated control, t test). Results are expressed as means ± standard deviations, n= 3. Similar results were obtained by using cells from another subject.
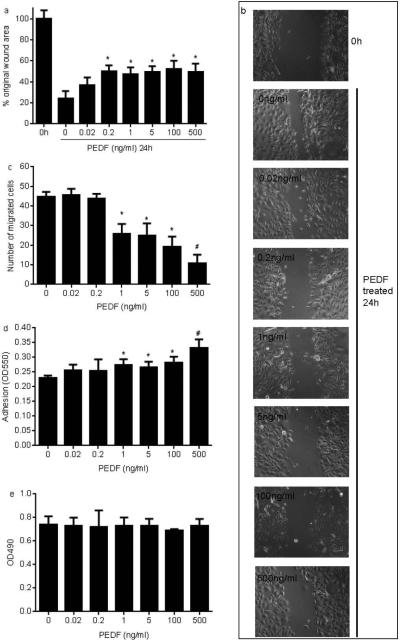
PEDF has previously been shown to inhibit migration and proliferation in the human keratinocyte cell line HaCAT (15) and in endothelial cells (1); therefore, we wondered if PEDF would have similar effects on primary NHEK. Using a scratch wound assay on a monolayer of NHEK, cells were treated with exogenous purified recombinant human PEDF. The results revealed that treatment with PEDF at concentrations ranging from 0.2-500ng/ml significantly inhibited NHEK migration in the scratch assay (Fig. 2a&b). This effect was further examined using a transwell migration assay. PEDF had a dose-dependent inhibitory effect on migration in the transwell migration assay (Fig. 2c), providing additional evidence that PEDF negatively affects keratinocyte migration. Furthermore, a similar exposure to PEDF resulted in a significant increase in the in vitro adhesion capacity of NHEK (Fig. 2d). In contrast, PEDF treatment did not affect NHEK proliferation (data not shown).
Figure 2.
PEDF inhibits NHEK migration and increases adhesion. a,b) Scratch migration assay: NHEK monolayers were treated with mitomycin C for 2 hours followed by the production of mechanical scratches and incubation with or without PEDF (0.02-500ng/ml) for 24 hours. The percent of the unoccupied area in the wounds was calculated compared to the time 0 untreated value (*p<0.01-0.001 compared to untreated control/0ng/ml, t test). c) Transwell migration assay: NHEK (1×105) were added in an upper chamber of 24-well transwell plate; lower chambers contained culture medium with a range of concentrations of PEDF. Following a 4 hour incubation period, the number of cells that had migrated was counted. d). Adhesion assay: NHEK (1x105) were cultured in a 96-well plate treated PEDF for 4 hours. The unattached cells were washed and adherent cells were fixed in ethanol, stained by crystal violet and then lysed by Triton x-100. The optical density at 550 nm was measured. (*p<0.001 compared to untreated control using at test). e) Proliferation assay: 5×103 NHEK cells/well were cultured in 96-well plate. After 24 hours, PEDF (0.02-500ng/ml) was added and the cells were then incubated for further 24 hours. Cell proliferation was examined using CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega). For all experiments of Figure 2, results are expressed as means ± standard deviations, n=3. Similar results were obtained by using cells from another subject.
The above results suggest that the early decrease of PEDF that is observed in wounds may be due to the surge of inflammatory cytokines and/or mechanical disruption of the keratinocyte layer. As the healing process progresses into the proliferative phase, the inflammatory response subsides, and PEDF returns to its uninjured level. In the resolving wound, restored levels of PEDF may inhibit further wound angiogenesis and encourage vascular regression. PEDF may modulate epithelial migration and thus support the return to skin homeostasis. We speculate that secreted PEDF may play an important role in the dynamic reciprocity between the epidermal and dermal layers of a healing wound. Future studies regarding the in vivo role of PEDF are needed to fully understand its function in wound healing.
Conclusion
PEDF decreases in the early stage of skin wound healing and returns to normal levels in the later remodeling phase. Mechanical injury to human skin keratinocytes in vitro results in a decrease in PEDF expression. Inflammatory cytokines such as those found in wounds also cause a decrease in keratinocyte PEDF production in vitro. While an anti-angiogenic role for PEDF in wounds seems likely, the results here show that PEDF can also regulate keratinocyte migration via increased cellular adhesion. The current study suggests that PEDF may play multiple important roles in skin wound healing. Furthermore, the modulation of PEDF production in keratinocytes by inflammatory stimuli may have implications for the pathogenesis of various inflammatory skin pathologies.
Supplementary Material
Acknowledgements
LC and LAD designed the study. LC performed the experiments. LC and LAD wrote the article. This publication was supported by NIH Grant R01GM50875. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interests
The authors state no conflicts of interests.
References
- 1.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science (New York, NY) 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 2.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. Journal of cellular biochemistry. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 3.Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends in molecular medicine. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ek ET, Dass CR, Choong PF. Pigment epithelium-derived factor: a multimodal tumor inhibitor. Molecular cancer therapeutics. 2006;5:1641–1646. doi: 10.1158/1535-7163.MCT-06-0107. [DOI] [PubMed] [Google Scholar]
- 5.Abe R, Yamagishi S, Fujita Y, et al. Topical application of anti-angiogenic peptides based on pigment epithelium-derived factor can improve psoriasis. Journal of dermatological science. 2010;57:183–191. doi: 10.1016/j.jdermsci.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Francis MK, Appel S, Meyer C, Balin SJ, Balin AK, Cristofalo VJ. Loss of EPC-1/PEDF expression during skin aging in vivo. The Journal of investigative dermatology. 2004;122:1096–1105. doi: 10.1111/j.0022-202X.2004.22510.x. [DOI] [PubMed] [Google Scholar]
- 7.Dong YH, Li ZQ, Sun Y, Zhuang L, Wang YK, Sun Q. Downregulation of pigment epithelium-derived factor in condyloma acuminatum. The Journal of international medical research. 2013;41:365–370. doi: 10.1177/0300060513476584. [DOI] [PubMed] [Google Scholar]
- 8.Orgaz JL, Ladhani O, Hoek KS, et al. ‘Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma’. Oncogene. 2009;28:4147–4161. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broadhead ML, Becerra SP, Choong PF, Dass CR. The applied biochemistry of PEDF and implications for tissue homeostasis. Growth factors (Chur, Switzerland) 2010;28:280–285. doi: 10.3109/08977191003604513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ipina Z, Lussier JG, Theoret CL. Nucleotide structure and expression of equine pigment epithelium-derived factor during repair of experimentally induced wounds in horses. American journal of veterinary research. 2009;70:112–117. doi: 10.2460/ajvr.70.1.112. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. Positional differences in the wound transcriptome of skin and oral mucosa. BMC genomics. 2010;11:471. doi: 10.1186/1471-2164-11-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feezor RJ, Paddock HN, Baker HV, et al. Temporal patterns of gene expression in murine cutaneous burn wound healing. Physiological genomics. 2004;16:341–348. doi: 10.1152/physiolgenomics.00101.2003. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Guo S, Ranzer MJ, DiPietro LA. Toll-like receptor 4 has an essential role in early skin wound healing. The Journal of investigative dermatology. 2013;133:258–267. doi: 10.1038/jid.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. Journal of immunology (Baltimore, Md : 1950) 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 15.Li CM, Li W, Man XY, et al. Pigment epithelium-derived factor plays an inhibitory role in proliferation and migration of HaCaT cells. Molecular biology reports. 2011;38:2099–2105. doi: 10.1007/s11033-010-0336-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.