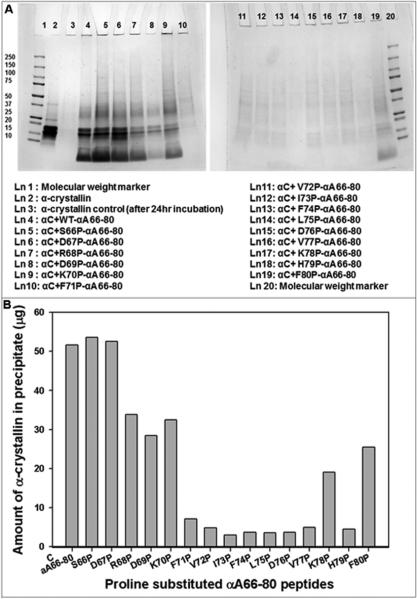
Figure 3.
A. SDS-PAGE of redissolved pellets of α-crystallin (αC) samples incubated with WT αA66-80 and proline-substituted αA66-80 peptides. α-Crystallin (100 μg) and peptides (each 50 μg) in 500 μl of 50 mM phosphate buffer ( pH 7.2) were incubated for 24 hrs at 37°C. After incubation, samples were centrifuged at 8000 rpm for 30 min. Pellets were redissolved in 20 μl of urea (6 M) and diluted to a total volume of 100 μl with 50 mM phosphate buffer (pH 7.2). A 25 μl aliquot of each sample was mixed with sample buffer and run in 4-20% SDS-PAGE. Lane 3 shows pellet formed after 24 hr incubation of α-crystallin by itself. Lane 4 to lane 19 represents the pellets formed after 24 hr incubation of α-crystallin with peptides. B. Aggregation of α-crystallin in the presence of proline-substituted αA66-80 peptides. The intensity of SDS-PAGE protein bands from figure 3 A were quantified using Image Lab Software (Bio-Rad). The band intensity values are converted to microgram based on standard graph constructed with band intensity for 2-15 μg of α-crystallin.