Figure 8. Proposed model for CD300f-mediated phagocytosis of AC.
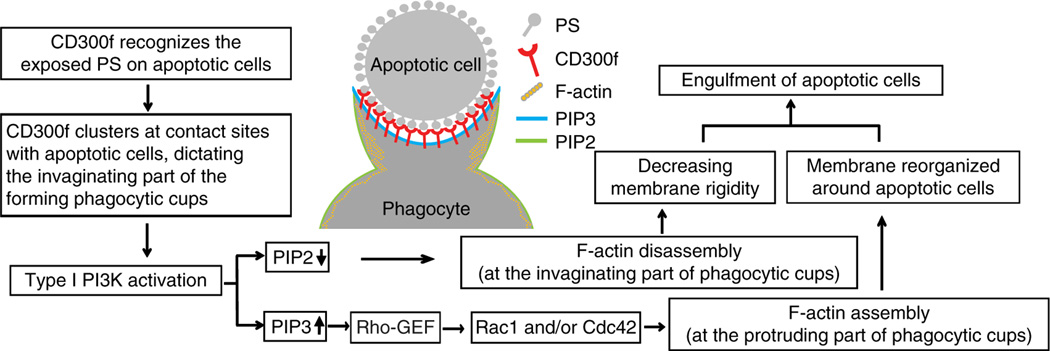
During the encounter of a phagocyte with an AC, CD300f recognizes and binds PS exposed on the surface of AC, and accumulates at the nascent phagocytic cup sites, dictating the invaginating part. This leads to phosphorylation of CD300f, predominantly on the tyrosine 276, and recruitment of the regulatory subunit of PI3K, p85α. In turn, the activated PI3K catalyses the conversion of PI(4,5)P2 (PIP2) to PI(3,4,5)P3 (PIP3). The PI3K-mediated decrease in the PIP2 levels at the invaginating part of phagocytic cups leads to a local cortical actin depolymerisation and/or generation of actin meshwork hypo-density, and thus decreasing membrane rigidity and removing a physical barrier that could impair the engulfment of apoptotic cell. At the same time, the PI3K-induced increase of PIP3 levels promotes activation of Rac1/Cdc42 likely via the Rho-GEFs (guanine nucleotide exchange factors), leading to the stimulation of actin polymerization along the protruding part of the phagocytic cups. This drives the membrane reorganization around AC and results in phagocytic cup closure and AC engulfment. Thus, the CD300f-mediated signal promotes the phagocytosis of AC through changes in the levels of phosphoinositol second messenger system that leads to activation of proteins involved in actin cytoskeleton rearrangements and, consequently, local changes in actin organization and membrane dynamics at the phagocytic cups.
