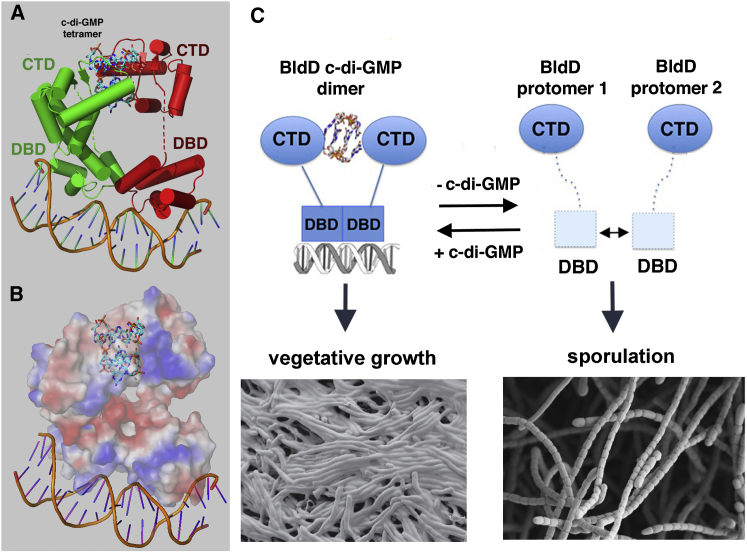
Figure 7.
The Molecular Mechanism of c-di-GMP-Activated DNA Binding by BldD and Its Control of Streptomyces Development
(A) Structure of the S. venezuelae BldD-(c-di-GMP)-DNA complex. One protomer is colored red and the other green. The c-di-GMP molecules are shown as sticks and the DNA as a cartoon. The linker region between the DBD and CTD (red or green dashed lines) is disordered in both protomers, indicating their conformational flexibility.
(B) Electrostatic surface representation of the BldD-(c-di-GMP)-DNA structure shown in the same orientation as (A).
(C) Schematic model of c-di-GMP-mediated activation of high affinity DNA binding by BldD, leading to repression of Streptomyces development. The BldD CTD is shown as ovals and the DBD as squares. The DBDs interact only weakly in vivo (indicated by the double-headed arrow). Increased c-di-GMP levels lead to BldD CTD dimerization, resulting in a significant increase in the local concentration of the DBDs, allowing them to dimerize optimally in the presence of cognate DNA to effect high affinity DNA binding. This leads to repression of the BldD regulon, thus blocking multicellular differentiation.