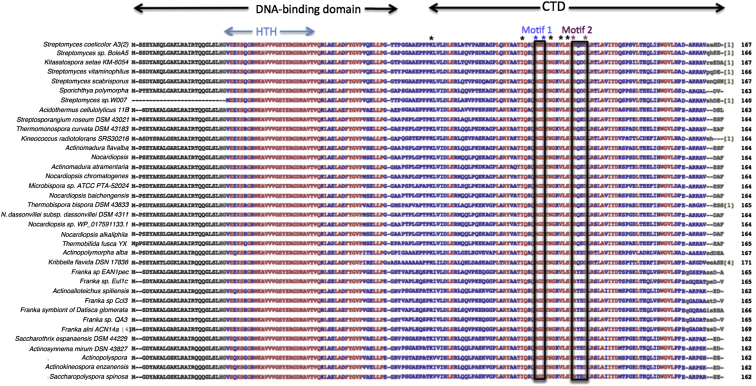
Figure S7.
Multiple Sequence Alignment Resulting from BLAST of the S. coelicolor BldD Protein, Related to Figure 5
Proteins that were > 95% identical were not included. Non-conserved residues are colored gray, highly conserved residues are blue and residues that are identical are colored red. S. coelicolor and S. venezuelae BldD share 100% identity within their DNA-binding domains; an alignment of their CTD sequences is shown in Figure 5A. The residues encompassing the DNA-binding domain and CTD are indicated as are the regions that correspond to the helix-turn-helix DNA-binding motif and Motifs 1 and 2 (blue and magenta) in the CTD. Key residues in Motif 1 (R114 and D116) and Motif 2 (R125 and D128) that contact the c-di-GMP are indicated by blue and magenta asterisks, respectively, and are completely conserved among the CTDs of all BldD proteins. Other residues that contact c-di-GMP are indicated by black asterisks. Such strong conservation within these actinomycetes suggests that BldD regulation via c-di-GMP is conserved across these organisms. The sequence alignment is better viewed at 300%.