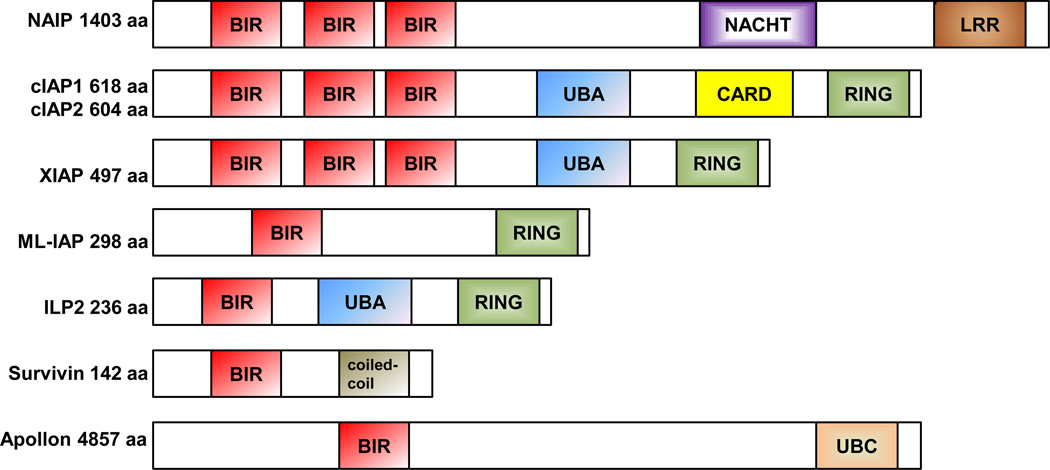
Figure 3. The IAP protein family.
The IAP family has eight members: NAIP, cIAP1, cIAP2, XIAP, ML-IAP, ILP2, survivin, and Apollon. All of the IAP proteins have a baculoviral IAP repeat (BIR) domain, which mediates protein-protein interactions. The RING domain mediates E3 ubiquitin ligase activity. Ubiquitin-associated domain (UBA) binds monoubiquitin and polyubiquitin chains. CARD is a caspase recruitment domain. UBC is an ubiquitin-conjugating domain. LRR is a leucine-rich domain. NACHT, a predicted nucleoside-triphosphatase domain, is named for its components: NAIP, CIITA (MHC class II transactivator), HET-E (20-hydroxyeicosatetranoic acid synthase), and TP1 (transition protein 1). The panels representing the proteins are not drawn to scale, and the amino acid lengths shown represent the canonical size. Figure is modified from [11]. Permission to reprint modified figure was obtained from Nature Publishing Group.