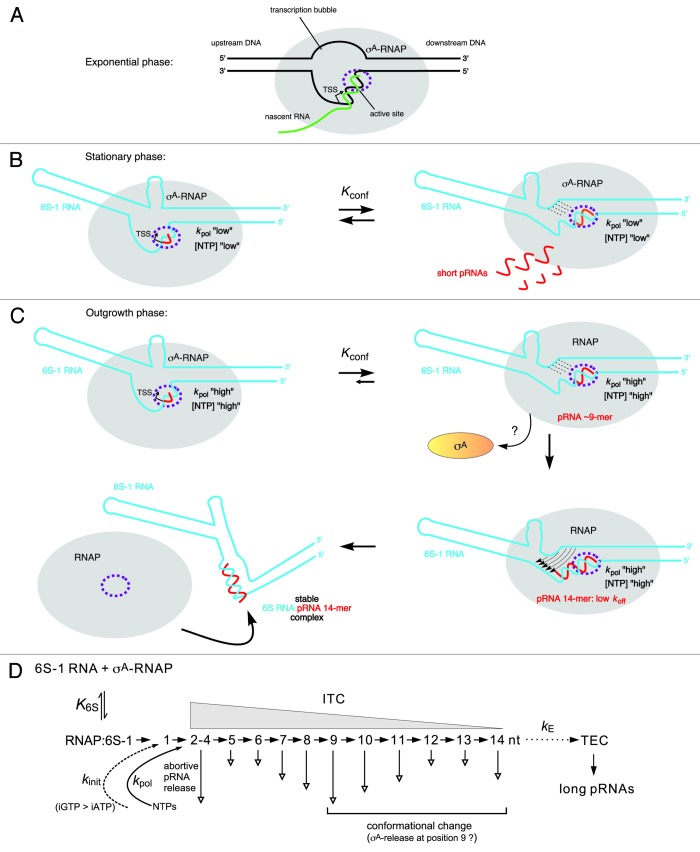
Figure 4. Model of the pRNA length-controlled structural rearrangement of B. subtilis 6S-1 RNA and its release from σA-RNAP, including mechanistic components inferred from the E. coli system. (A) In exponential phase, cellular 6S-1 RNA concentrations are low, only small amounts of pRNAs are synthesized and σA-RNAP is predominantly involved in gene transcription. The sketch illustrates a complex of RNAP and open promoter DNA; the latter is thought to be mimicked by 6S RNA.3 (B) 6S-1 RNA levels raise in late exponential and stationary phase and a larger fraction of σA-RNAP is sequestered in complexes with 6S-1 RNA. In late stationary phase, nutrients including NTPs are scarce and abortive transcripts/short pRNAs dissociate instead of being elongated to longer pRNAs because the rate constant for nucleotide addition is relatively slow (kpol “low”). Here, 6S-1 RNAs begin to transiently rearrange their structure (right panel), but fall back to the ground state (left panel) owing to pRNA dissociation; it is predicted that 6S-1 RNA molecules oscillate between their ground state and the transiently rearranged structure; if this also involves σA release and rebinding to RNAP (depicted in panel C) as suggested for the E. coli system upon pRNA 9-mer synthesis, remains to be seen. (C) In a newly initiated exponential growth phase (outgrowth), nutrients are resupplied and NTP levels increase. As a consequence, kpol increases (kpol “high”) and the fraction of longer pRNAs rises (12 to 14-mers), which stably rearrange 6S-1 RNA structure and trigger release of RNAP. An increase in the fraction of longer pRNA ~14-mers during outgrowth in vivo has been detected by dRNA-seq.5 Since the sigma factor dissociates from RNAP upon synthesis of a pRNA 9-mer in the E. coli system, we have tentatively included this mechanistic element also for the B. subtilis system. An open question is if the 6S-1 RNA rearrangement is already complete after synthesis of a pRNA 9-mer (as in the E. coli system) or incomplete until a stable RNAP:pRNA ~14-mer complex is formed. In the first three sketches, the hairpin in the 3′-CB is shown with a curved stem to indicate its equilibrium with an open structure. The two helical arms are shown as slightly bent to consider that formation of the hairpin constricts this side of the central bulge. In the top sketch on the right, stippled lines depict the progressive disruption of helix P2; thin arrows in the bottom sketch on the right illustrate formation of the central bulge collapse helix (see Fig. 1 B). The fully rearranged 6S-1 RNA structure is depicted in the bottom sketch on the left; the orientation of the helical elements is not known and thus arbitrary. (D) Kinetic scheme for pRNA synthesis in B. subtilis, adapted from abortive transcription initiation at DNA promoters.50,73 Ground state binding of 6S-1 RNA to σA-RNAP is governed by the equilibrium constant K6S. Initiation of pRNA synthesis depends on the rate constant for initiation, kinit, which has been shown to be faster with GTP than ATP in B. subtilis, but not in E. coli.49 With pRNA polymerization, the RNAP:6S-1 RNA complex acts as an initial transcribing complex (ITC) releasing abortive transcripts of various length (here mainly 2–14 nt) to different extents (indicated by the length of the vertical arrow; inferred from dRNA-seq data using poly(A)5 and poly(C) tailing (unpublished data) of cellular RNAs. Only pRNAs of ≥ 12 nt stably rearrange the 6S-1 RNA structure owing to their sufficiently low koff. The rate constant kE describes the transition from an ITC to a productive transcription elongation complex (TEC) and may be of limited relevance to pRNA transcription in vivo.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.