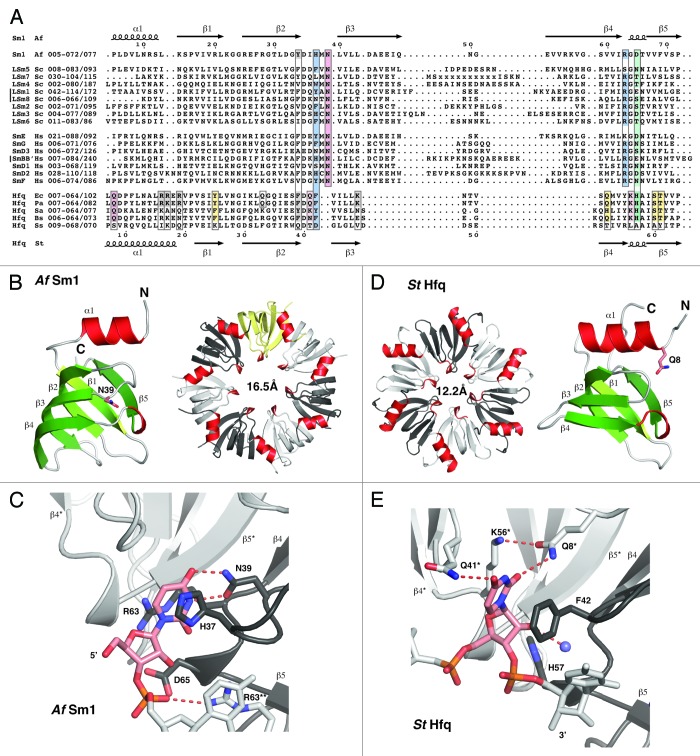
Figure 1. Alignment and structure of crystallized ring-forming (L)Sm and Hfq proteins. (A) Structure-based sequence alignment. Secondary structure elements and numbering above and below the alignment are from the AfSm1 and StHfq proteins, respectively. Only the crystallized (L)Sm-core of each protein is aligned. The numbers preceding each sequence refer to the aligned residues and to the total length of each protein. They also indicate the presence and size of C-terminal extensions. The crystallized sequences of StHfq and EcHfq are identical. Positions involved in RNA or nucleotide binding are boxed. Blue: Stacking partners for the nucleobase in the proximal site. Hydrogen bonds to the RNA backbone in the case of R63 and its (L)Sm homologs. Red: Base recognition by hydrogen bonds in the proximal site. Green: Constriction of the RNA backbone in the proximal site and RNA 3′-end recognition in the case of Hfq. Yellow: Conserved “R”-site on the distal surface of Hfq; Q33 in EcHfq: “A”-site on the distal surface of EcHfq, not present in many gram-positive bacteria. Gray: Conserved uridine-site on the outer rim of the proximal surface of Hfq and of archaeal LSm proteins, plus members of the basic patch on the lateral surface of StHfq and EcHfq (R16, R17, R19, K47) that is less well conserved in gram-positive bacteria. Sequence information is from the respective PDB files listed by PDB-ID, chain-ID, resolution, and species: Sm1, 1i4k_A,9 2.50 Å, Archaeoglobus fulgidus, Af; LSm1–7, 4c92_ABCDEFG,84 2.30 Å, and 4m75_A,85 2.95 Å, Saccharomyces cerevisiae, Sc; LSm8, 4m77_A,86 3.11 Å, Saccharomyces cerevisiae, Sc; Sm proteins, 2y9a_ABCDEFG,87 3.60 Å, Homo sapiens, Hs; EcHfq, 2ylc_A,29 1.30 Å, Salmonella typhimurium, St and Escherichia coli, Ec; PaHfq, 4j6y_A,28 2.14 Å, Pseudomonas aeruginosa, Pa; SaHfq, 1kq2_A,26 2.71 Å, Staphylococcus aureus, Sa; BsHfq, 3ahu_A,39 2.20 Å, Bacillus subtilis, Bs; SsHfq, 3hfo_A,79 1.30 Å, Synechocystis ssp., Ss. (B) LSm monomer and homoheptamer. The monomer of the archaeal AfSm1 protein9 is shown with α-helices in red and β-strands in green. N39 (sticks) marks the nucleotide binding pocket shown in (C). The loop L(β4-β5) forms a short 3–10 helix that is highly conserved. The protomers in the heptameric ring are colored with in white, gray and yellow, and helices in red for orientation. The diameter of the pore is 16.5 Å (proximal view), using the Cβ atom of D65 as a reference. (C) Nucleotide binding in the proximal site of an LSm protein. Uridine recognition is shown for AfSm1,9 with optimal geometry for hydrogen bonds (dotted red). Important side chains are shown as sticks with nitrogens in blue and oxygens in red. The asterisk and double asterisk mark the preceding and following protomer in the 5′-3′ direction of the bound RNA, respectively. (D) Hfq monomer and homohexamer. Compared with the AfSm1 protein, the monomer of the bacterial StHfq protein29 reveals an extended α-helix α1, a loop L(β2-β3) that has a distinct structure, and a variable loop L(β3-β4) that is very short. Q8 (sticks) marks the modified nucleotide binding pocket shown in (E). The diameter of the pore is 12.2 Å (proximal view), using the Cβ atom of H57 as a reference. (E) Nucleotide binding in the proximal site of Hfq. Uridine recognition by hexameric StHfq29 is structurally distinct from (L)Sm proteins and involves residues from two neighboring protomers. Base recognition geometry is rather poor, a potential consequence of the constricted RNA backbone conformation. A conserved water molecule is shown as a blue sphere (see also Fig. 2). H57 is positioned close to the 3′-oxygen of the ribose. The alignment was done with the help of ESPRIPT,110 structural analysis and figures were done with the help of COOT111 and PYMOL (http://www.pymol.org).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.