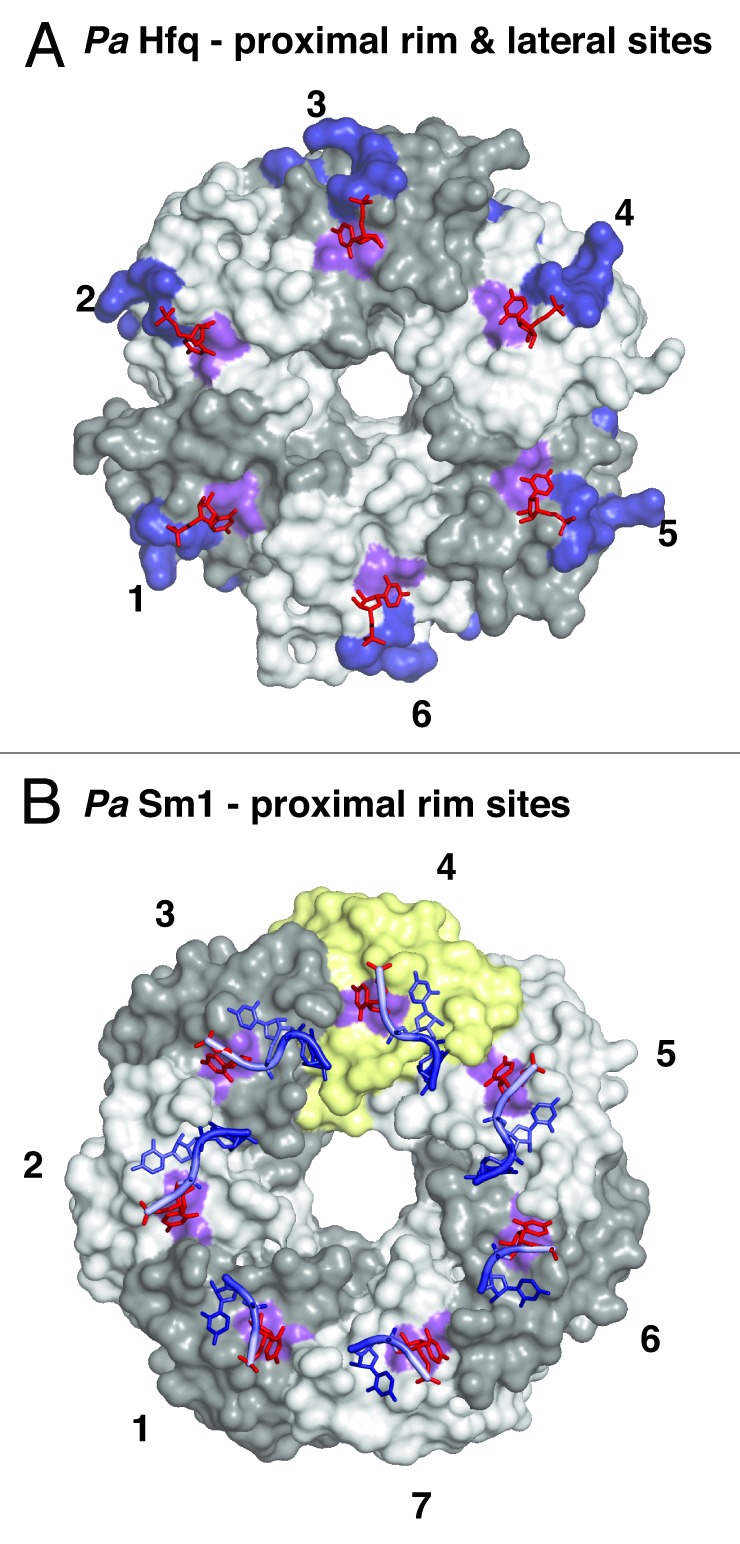
Figure 4. Additional RNA binding surfaces on Hfq and (L)Sm proteins. (A) RNA binding on the outer rim of the proximal surface and on the lateral surface of Hfq. Transparent surfaces of Hfq protomers are colored in white and gray and numbered for orientation. A conserved uridine binding site on the proximal surface of bacterial PaHfq (Pseudomonas aeruginosa) is colored in purple (corresponding to F39 in EcHfq).28 The areas corresponding to the basic patch on the lateral surface of StHfq are colored in dark blue (corresponding to R16, R17, R19, K47 in StHfq and EcHfq).29 The basic patch allows the specific recognition of regulatory sRNAs and catalyzes base-pair formation and exchange. The uridines co-crystallized with PaHfq are shown as red sticks. (B) Conservation of the uridine binding site on an archaeal LSm heptamer.53 The surface of the additional protomer in the homoheptameric ring of the archaeal PaSm1 (Pyrococcus abyssii) protein is shown in yellow. The conserved uridine binding site is colored in purple (corresponding to Y34 in AfSm1) and the bound uridines are shown as red sticks. Other nucleotides and the RNA backbone of the co-crystallized oligomers are drawn in blue as described before.
