Abstract
Effectiveness of fermentation using lactic acid bacteria (LAB) for recovering lipids and proteins simultaneously from freshwater fish head (FWH) was evaluated. Three different proteolytic LAB (Pediococcus acidilactici NCIM5368, Enterococcus faecium NCIM5335 and Pediococcus acidilactici FD3) isolated from fish processing wastes were employed in the fermentation process. The fermentation conditions involved 10 % (w/w) glucose, 2 % (w/w) NaCl and 10 % (v/w) LAB cultures at 37 °C. The process resulted in 38.4 % of degree of hydrolysis (in case proteins) and a recovery of 63.6 % of the oil present in the material. The fermentation process did not affect the fatty acid profile of lipids. The hydrolyzed protein rich fermentation liquor exhibited DPPH radical scavenging activity (EC50 – 5.1 mg protein) as well as antagonistic properties towards several bacterial and fungal pathogens. The minimum inhibitory concentrations (MIC) of fermentated liquor (with E. faecium NCIM5335 as starter) were 10 and 12 mg/ml for Listeria monocytogenes and Salmonella itridicus, respectively. A higher MIC (60 and 96 mg/ml for Aspergillus ochraceus and Penicillium chrysogenum, respectively) was observed in case of fungal pathogens. Both the oil and protein hydrolysate rich liquor from fish head can be used as biofunctional ingredients in both human food as well as livestock feed formulations.
Keywords: Fish head, Lactic acid bacteria, Lipids, Protein, Antioxidant, Antimicrobial
Introduction
Fish heads (FH) are the major edible by-products generated from fresh water fish processing industries, which are rich in lipids and protein. The fish production from freshwater bodies through aquaculture currently stands in close to 33 million metric tonnes, accounting for >22 % of world fish production (FAO 2010). Considering the fact that fresh water FH are a rich source of protein and polyunsaturated lipids (Swapna et al. 2010) and constitute about 15–25 % (depending on the species) of the fresh water fish biomass, it can be estimated that freshwater fish processing waste in the form of head alone amounts to >4.5 MMT. Hydrolysis processes have been developed to convert under-utilized fish and fish byproducts into a marketable and acceptable form, which can be widely used in food rather than as animal feed or fertilizer. Lactic acid fermentation (LAF) being an eco-friendly method results in biological silage wherein lactic acid bacteria (LAB) generate acid in situ for preservation of waste or for recovery of biomolecules from by-product. In addition to acid, LAB also produce proteases for the hydrolysis of protein (Jini et al. 2011) and some of the lactobacilli produce antimicrobial compounds, which increase the preservation effect. The process of LAF has been employed to recover various biomolecules such as protein, lipids, carotenoids and chitin from among host of biomolecules (Amit et al. 2011). LAF has been studied for the simultaneous recovery of lipids and protein from fish visceral waste (Amit et al. 2011) but that can only be used for aquaculture and animal feed.
The lipids from these edible wastes are rich in polyunsaturated fatty acids that can be used for human consumption. Economic value of fish oil had a record increase in the past few years due lopsided demand–supply issues; and, is expected to continue to increase in the near future (Turchini et al. 2009). In order to offset the lesser supply and higher demand, there is a great urgency to find and implement sustainable alternatives to fish oil. Lipids recovered from fish head can be better alternative to fish oil and till some extent reduce the demand of fish oil production. Protein hydrolysate from fish muscle (Rajaram and Nazeer 2010) and visceral waste (Amit et al. 2011) have been studied for antioxidant and antibacterial activity. Natural antioxidant such as ascorbic acid and α-tocopherol are preferred over synthetic antioxidant due to their safety regard to health. In order to develop natural and safer antioxidant having nutritional value synergistic effect of amino acids, peptides and protein hydrolysate has been attracting research attention considerably. Similarly, protein hydrolysate after LAF can be used as a probiotic; and, use of probiotic as food additives is preferred over that of antibiotics as they do not exhibit any of the undesirable effects associated with the use of antibiotics viz, toxicity, allergy, bacterial drug resistance (Ajitha et al. 2004). In our present study, we assessed the effectiveness of LAF for the simultaneous recovery of lipids and protein from fresh water FH. The LAB used for the fermentation of fish head possesses good proteolytic properties and produces bacteriocin having a broad antibacterial spectrum (Amit et al. 2009; Jini et al. 2011). Further, the fatty acid profile of recovered fish oil along with the antibacterial and antioxidant activities of the hydrolyzed protein rich liquor portion was also analyzed, in order to explore its possible potential as a high value ingredient in human food.
Materials and methods
Materials
Fresh water fish heads (FWFH) were obtained by dressing of freshly harvested Indian major carps (Rohu and Catla) procured from the local market and transported to laboratory under iced conditions. DPPH (2, 2′-diphenyl-1-picrylhydrazyl), was purchased from Sigma-Aldrich Chemie (Steinheim, Germany). LAB isolates namely Enterococcus faecium NCIM5335, Pediococcus acidilactici FD3, Pediococcus acidilactici NCIM5368 and all the pathogens were obtained from the institute culture collection. All microbiological media were procured from Hi-Media (Hi-Media, Mumbai, India). All the solvents and chemicals were of analytical grade.
Preparation of silage
FWFH were crushed into small pieces using bowl chopper (TC11, Scharfen 58413, West Germany) followed by homogenization in a blender (Stephan Mill; Stephan UM, Germany) for 5 min. The homogenized mass was mixed and cooked under steam for 20 min. After cooling, 10 % (w/w) dextrose and 2 % (w/w) salt were added and mixed thoroughly. The LAB cultures E. faecium NCIM5335, P. acidilactici FD3, P. acidilactici NCIM5368 were grown in 100 ml of MRS-Broth (Hi-Media, India) for 24 h at 37 °C in a shaking incubator (Technico Ltd., India) set at 100 rpm. The cells were harvested by centrifuging (C31 Cooling centrifuge, Remi-India, India) at 3000 × g for 10 min, washed twice with sterile physiological saline and resuspended in physiological saline (100 ml). The silage mix was placed in airtight container and incubated for 72 h at 37 °C. After 24 h of fermentation the content was centrifuged at 5000 × g for 20 min and upper oil layer was collected. The lower FWH fermented protein rich hydrolysate (FHPH) was analyzed for degree of hydrolysis (DH) and antioxidant activities.
Yield, quality and fatty acid composition of lipids recovered on fermentation
Oil yield after fermentation of cooked homogenized FWFH was calculated as % of total lipid content in FWFH (Amit et al. 2011). Total lipid content in the FWFH as determined by the method of Bligh and Dyer (1959) was considered as 100 %; and, the yield of lipids on fermentation was computed as -
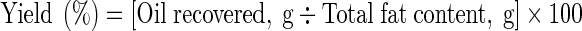 |
Acid and peroxide value of the recovered oil was estimated as per AOAC (2000) to ascertain the quality of oil. Fatty acid composition of recovered lipids was determined by preparing the fatty acid methyl esters (FAME) of the recovered oil. Briefly, oil samples were transmethylated using 2 M methanolic sodium hydroxide followed by 2 M methanolic hydrochloric acid to obtain FAME. FAME were analysed by using a gas chromatography (GC) system (Shimadzu GC 2014; M/s Shimadzu, Kyoto, Japan) fitted with a flame ionization detector (FID) for identifying the individual fatty acids. FAME dissolved in hexane were analyzed using a fused silica capillary column (30 m × 0.32 mm × 0.25 μm) (Omegawax™ 320; M/s Supelco, Bellefonte, USA) with a split ratio of 1:30). The temperatures (°C) of injector, column and detector were set at 250, 200 and 260, respectively.
Degree of protein hydrolysis
DH of the FHPH resulted after lactic acid fermentation of FH was estimated as per the methodology described by Amit et al. (2011) and was computed as - DH (%) = [10 % TCA soluble N2 in the sample ÷ Total N2 in the sample] × 100.
Antioxidant activities of fermented head protein hydrolysate
Preparation of sample for in-vitro antioxidant and antibacterial assays
The FHPH obtained was filtered using muslin cloth to obtain filtered fermented liquor (FFL). FFL was further centrifuged at 5000 × g for 20 min to obtain a sediment-free supernatant. This supernatant was referred to as centrifuged FFL (CFFL). CFFL was lyophilized and the lyophilized sample was used for antioxidant and antimicrobial properties. The in-vitro antioxidant assays included total antioxidant activity (TAO) and DPPH radical scavenging activity.
Total antioxidant activity
TAO was carried out as per the method described by Amit et al. (2011). TAO of CFFL was determined by mixing sample with 3 ml of reagent solution [3.0 ml; 0.6 M sulfuric acid: 28 mM sodium phosphate: 4 mM ammonium molybdate (1:1:1 v/v/v)]. Reaction mixture was incubated in a water bath at 95 °C for 90 min, cooled to room temperature and the absorbance was measured at 695 nm. TAO was expressed as of μg of ascorbic acid equivalents (AAE) per mg of protein in fermented hydrolysed sample.
Diphenyl picrylhydrazyl (DPPH) radical scavenging activity
The DPPH radical scavenging capacity of CFFL was determined by the method previously described in Amit et al. (2011). Briefly, 100 μl of sample and made up to 2 ml with distilled water followed by addition of 2.0 ml of 0.16 mM DPPH solution (in methanol). The mixture was vortexed and kept at room temperature for 30 min in the dark. Sample blank was prepared by replacing DPPH with methanol. The absorbance of all the sample solutions was measured at 517 nm. Methanol along with DPPH served as the control. The scavenging activity (%) was calculated by using the formula:
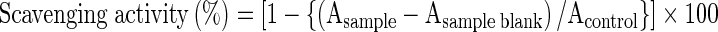 |
Antibacterial and antifungal susceptibility assay
Microorganisms
Bacterial strains such as Bacillus cereus F4810, Bacillus subtilis MTCC441, Staphylococcus aureus FRZ722, Streptococcus pyogenes MTCC1923, Listeria monocytogenes Scott-A, Escherichia coli MTCC118, Pseudomonas aeruginosa MTCC741, and Yersinia enterocolitica MTCC859, Salmonella typhi FB231, S. paratyphi FB254, S. itriditicus FB256, Micrococcus luteus and fungal strains Aspergillus flavus MTCC277, A. niger MTCC218, A. ochraceus MTCC4643, A. oryzae MTCC262, Fusarium oxysporum MTCC1755 and Penicillium chrysogenum MTCC616 were obtained from Microbial Type Culture Collection (MTCC), Chandigarh, India and culture collection center maintained at CFTRI, India.
Preparation of spore suspension
All the fungal cultures were grown on potato dextrose agar (PDA) slants at 25 °C until the spores ramified (7–10 days). Spores were suspended by adding Tween 20 solution (0.1 % v/v) in distilled water and inoculated viable spores (106) per mL.
The antibacterial activity was carried out using agar well diffusion method (Denton and Kerr 1998). The 18–24 h old bacterial strains were grown on nutrient broth and incubated at 37 °C under shaking condition. The culture broth of 0.1 mL containing 108–109 cfu/mL was spread on nutrient agar plate (15 cm) by spread plate method. Wells were bored (8 mm diameter) in the agar plates and filled with protein hydrolysate and were incubated at 37 ±1 °C for 24 h and 48 h. Tetracycline (10 μl/ml) was used as positive control. The zone of growth inhibition was expressed in mm.
The antifungal properties of protein hydrolysate were studied against various fungi (Singh et al. 2004). The known concentrations of the CFFL were added onto the plate along with the PDA before plating and the content was evenly mixed. Point inoculation of the respective fungi was carried out. The PDA plate without CFFL was considered as control. The plates were incubated at 27 ±1 °C temperature for 5–7 days. Nystatin (1 μl) was used as the positive antifungal agent. At the end of the incubation period, the susceptibility of the test organisms was determined by measuring the mean growth values and were converted into percentage of mycelial growth inhibition (MGI) in relation to the control treatment, by using formula MGI (dc-dt)/dc X100, where dc and dt represent mycelial growth diameter in control and treated petriplate, respectively. All the experiments were carried out in triplicates.
Determination of Minimum Inhibitory Concentration (MIC) for bacteria and fungi
The MIC of FHPH against selected bacterial strain were carried out according to Thongson et al. (2004). Different concentrations 5, 10, 15, 20, 40, 60, 80, 100 μl (protein concentration 7–8 mg/ml) of the CFFL and 100 μL of the bacterial suspension (105 CFU/ml) were added aseptically to 10 ml of nutrient broth separately and incubated for 24 h at 37 °C. Growth was observed both visually and by measuring the optical density (OD) at 600 nm. MIC was defined as the lowest concentration which resulted in a reduction of >90 % in the observed absorbance. Likewise, the MIC of the fungal strains was carried out using different concentration (5 to 100 μl) of CFFL (protein concentration 7–8 mg/ml) on A. ochraceus and Penicillium sp using potato dextrose broth. These were incubated at 27 °C for 4–5 days and observed for the growth inhibition. Triplicate sets of tubes were maintained for each concentration of test sample.
Scanning electron microscopy (SEM)
To determine the efficacy of protein hydrolysate on the morphology of the cells, SEM studies were performed on L. monocytogenes and S. itriditicus treated with MIC of protein hydrolysate. Controls were prepared without protein hydrolysate. The bacterial samples were washed gently with 50 mM phosphate buffer solution (pH 7.3), fixed with 2.5 g/100 ml glutaraldehyde. The specimen was dehydrated using sequential exposure per ethanol concentrations ranging from 30–100 %. After dehydration, the samples were spread on a double sided conducting adhesive tape pasted on a metallic stub, subjected to gold covering, and observed under a scanning electron microscope (LEO 435VP, UK) at 20 kV attached to Video copy processor (Mitsubishi, Japan) . Photographs were taken using 35 mm camera (Ricoh, Japan) that was connected to monitor optically through fibre optics.
Three-day-old fungal cultures of Penicillium sp. and A. ochraceus on PDA treated with sterile filter paper discs (10 cm diameter) soaked in protein hydrolysate placed on the inner surface of the Petri dish lid. Then, the dishes were sealed with parafilm and incubated upside-down at 25 °C, and negative control without protein hydrolysate was used for SEM to study the mode of action of the protein. The segments measuring 5–10 mm were cut at periphery of the colony from the cultures growing on the PDA plates and were placed in vials containing 3 % glutaraldehyde in phosphate buffer saline (PBS) (pH 7.4) at 4 °C and further processed as mentioned for bacterial cells.
Statistical analysis
All the experiments were comducted in triplicates, wherever required. The data was subjected to analysis of variance to determine the significant difference between different treatments and mean separation was accomplished by Duncan’s multiple range test (Statistica 1999).
Results and discussion
Recovery of lipids and protein from fresh water fish head
Fresh water fish head (Rohu and Catla) had a lipid and protein content of 26.47 % and 55.23 % (both on dry weight basis), respectively. Oil recovery after fermentation of FWH using different LAB cultures is presented in Table 1. The total lipid content in FWH was 15–16 % (wwb). Oil recovery showed variations among different LAB cultures used. E. faecium NCIM5335 gave highest oil recovery of (63.6 %), followed by P. acidilactici NCIM5368 (58.3 %), the least being in P. acidilactici FD3 (39.2 %). Hence, it can be said that fermentation using LAB can be an effective approach for oil recovery from fresh water fish head.
Table 1.
Summary of lactic acid fermentation for simultaneous recovery of lipids and protein from fresh water fish head (n = 3)
| Attributes | E faecium NCIM5335 | P acidilactici NCIM5368 | P acidilactici FD3 |
|---|---|---|---|
| Lipid | |||
| Oil Recovery (%) | 63.6 ± 3.12a | 58.3 ± 3.87a | 39.2 ± 2.80b |
| Peroxide value | 37.5 ± 3.62a | 42.0 ± 2.71 a,b | 48.4 ± 3.63 b |
| Acid value | 7.8 ± 0.84a | 8.2 ± 1.14 a | 6.9 ± 0.40 a |
| Protein | |||
| DH (%) | 38.4 ± 2.18a | 29.0 ± 2.15b | 36.0 ± 4.18 a |
| TAO (mg AAE/mg) | 13.4 ± 1.17 a | 11.1 ± 0.83b | 11.3 ± 2.78b,c |
| DPPH (EC50) | 5.1 ± 0.42a | 7.2 ± 0.50b | 6.7 ± 0.92c |
Peroxide value - meqv oxygen kg−1 sample, Acid value - mg NaOH/g of sample
DH: Degree of hydrolysis, TAO – Total antioxidant activity; DPPH : DPPH radical scavenging activity: EC50 : effective concentration of protein (mg) for 50 % radical scavenging activity. AAE: Ascorbic acid equivalent. Values within the same row with the same superscripts are not singnificantly different (p > 0.05) (n = 3)
The oil recovered on fermentation using different LAB cultures was assessed for its quality which was determined with respect to acid value and peroxide value. The acid value (mg NaOH g-1 sample) in the oil recovered is presented in Table 1. Acid value of the recovered oil was in the range of 2.2–2.9 (mg NaOH g−1 sample) with the maximum in case of P. acidilactici FD3 (2.9) and P. acidilactici NCIM5368 (2.8). Acid value of oil recovered from fish head is lower compared to visceral waste, as viscera are rich in lipases (Nayak et al. 2004) and lipolytic bacteria. But lipase produced by LAB can increase the acid value of the oil. Due to the action of lipases, the lipids present in the fish viscera are hydrolyzed thus releasing the fatty acids, which contribute to acid value. Peroxide value indicates the degree of oxidation of lipids which is presented in Table 1. The peroxide value (meqv oxygen kg−1 sample) in the oil increased significantly from an initial value of 11.23 meqv oxygen kg−1 sample in fresh and was found to be maximum in case of P. acidilactici NCIM5368 and P. acidilactici FD3 (42 and 48.4 meqv oxygen kg−1 sample), respectively. Peroxide value can be reduced by the addition of synthetic antioxidant such as TBHQ to the homogenized head before fermentation. Changes in peroxide value, an indicator of lipid oxidation, during fermentation of fish viscera has been reported by Ahmed and Mahendrakar (1996) where slight increase was observed in peroxide value which later reduced due to breakdown of oxidized products. Further they also suggested the use of antioxidants to prevent oxidation of lipids and development of rancidity during storage of fermented fish viscera. The fermentation may also result in formation of peptides possessing antioxidant activity, which can prevent lipid oxidation (Amit et al. 2011).
Fatty acid composition of recovered oil from hydrolysed fish head waste is presented in Table 2. There was no significant difference in the fatty acid profile of the oil recovered on fermented homogenized head using different LAB cultures when compared to the fresh oil. Unsaturated fatty acids dominated the fatty acid profile of the recovered oil. There was no significant difference in the amount of saturated fatty acids like palmitic acid (C16:0) stearic acid (C18:0) as well as unsaturated fatty acids such as eicosapentaenoic acid (EPA) (C20: 5), palmitoleic acid (C16:1), oleic (C18:1) and linoleic (C18:3) (Table 2). This observation correlated with Gbogouri et al. (2006) wherein no difference in fatty acid profile of oil recovered from salmon heads by solvent extraction and enzymatic hydrolysis was noticed. Similarly, in case of fermentation of fresh water viscera using LAB, there was no change in fatty acid composition in the recovered oil as reported by Amit et al. (2011).
Table 2.
Fatty acid composition of oil recovered on fermentation of fresh water fish head using different lactic acid bacteria (n = 3)
| Head | Fresh | NCIM5335 | FD3 | NCIM5368 |
|---|---|---|---|---|
| C14:0 | 3.7 | 3.8 | 3.8 | 3.7 |
| C16:0 | 29.6 | 28.7 | 29.8 | 29.3 |
| C18:0 | 4.5 | 4.6 | 4.3 | 4.3 |
| C20:0 | 1.1 | 1.3 | 1.1 | 1.1 |
| ΣSFA | 36.4 | 39.8 | 40.4 | 38.5 |
| C16:1 | 13.1 | 12.6 | 13.3 | 13.3 |
| C18:1n-9 | 19.7 | 19.7 | 18.5 | 18.7 |
| C18:1n-7 | 2.5 | 3.9 | 3.6 | 3.5 |
| ΣMUFA | 35.3 | 36.2 | 35.5 | 35.5 |
| C18:2n-6 | 4.1 | 4.1 | 4.0 | 4.2 |
| C18:3n-3 | 5.7 | 5.5 | 5.6 | 5.7 |
| C20:4n-6 | 2.4 | 2.2 | 2.0 | 2.1 |
| C20:5n-3 | 2.8 | 2.6 | 2.6 | 2.7 |
| C22:5n-3 | 1.6 | 1.4 | 1.3 | 1.3 |
| C22:6n-3 | 2.9 | 2.5 | 2.9 | 3.2 |
| ΣPUFA | 19.1 | 18.3 | 17.2 | 19.1 |
SFA Saturated fatty acid, MUFA Mono unsaturated fatty acid, PUFA Poly unsaturated fatty acid. NCIM5335 - Enterococcus faecium NCIM5335, FD3 - Pediococcus acidilactici FD3, NCIM5368 - Pediococcus acidilactici NCIM5368; Fatty acid concentrations above 0.5 % are only considered for the above
Antioxidant activities of fermented protein hydrolysate
The protein hydrolysates obtained from wastes/by-products of animal and fish processing industry exhibit antioxidant activity (Amit et al. 2009; Bijinu et al. 2011; Sachindra and Bhaskar 2008). TAO and DPPH radical scavenging activity of CFFL obtained from FHPH by different LAB cultures is presented in Table 1. DPPH has been used extensively as a free radical to evaluate reducing substance and is a useful reagent for investigating the free radical scavenging activities of protein hydrolysates. DPPH radical scavenging activity expressed in EC50 which was found to be higher in case of E. faecium NCIM5335 (5.1 mg protein), least being in P. acidilactici NCIM5368 (7.2 mg protein). The method is based on the reduction of methanolic DPPH solution in presence of a hydrogen donating antioxidant due to formation of a non-radical form of DPPH-H by the reaction and this modification is visually noticeable as a discoloration from purple to yellow (Shon et al. 2003)
Total antioxidant activity (TAO) was expressed as mg of ascorbic acid equivalents per microgram of protein in CFFL (AAE/mg). E. faecium NCIM5335 showed highest TAO of (13.4 mg of AAE/mg of protein), followed by P. acidilactici FD3 showing (11.3 AAE/mg protein) and P. acidilactici NCIM5368 (11.1 AAE/mg protein). The TAO exhibited by the CFFL could possibly be due to the peptides and amino acids resulting from the hydrolysis of fish waste proteins. In this phosphomolybdenum method, molybdenum VI (Mo6+) is reduced to form a green phosphate/Mo5+ complex (Amit et al. 2009).
Antibacterial activity of FHPH
The in- vitro antibacterial susceptibility of the FHPH obtained on fermentation of homogenized fish head using different LAB showed antibacterial activity against bacterial pathogens (Table 3). The FHPH extract was found to be effective against Gram- positive bacteria such as S. aureus, L. monocytogenes, B. cereus, B. subtilis, M. luteus and Gram- negative pathogens such as E. coli, S. itriditicus, S. paratyphi, Y. enterocolitica and S. typhi. The inhibition zone using FHPH obtained using different LAB ranged from 13.5–29.75 mm and maximum inhibition was obtained with L. monocytogenes (29.75 ± 0.2) and S. itriditicus (28.25 ± 1.25). The antibacterial activity exhibited by the liquor portion rich in hydrolysed portion could possibly be because of presence of antimicrobial peptides (bacteriocin) produced during fermentation using LAB and peptides formed on hydrolysis of protein present in head. Kawai et al. (2007) have reported antagonistic property of bovine lactoferrin hydrolysate against mastitis pathogens. Similarly, hydrolysates of food proteins by gastrointestinal proteases have also been shown to be antibacterial as well as immunostimulatory in nature (Gediminas et al. 2006). Bactriocins are known to kill the pathogenic bacteria either by forming pores in the cytoplasmic membrane or by acting on the energized membrane vesicles to disrupt the proton motive force (PMF), inhibit uptake of amino acids, and cause release of accumulated amino acids (Abee et al. 1995). In case of P. acidilactici FD3 FHPH, maximum inhibition was observed with S. aureus (29.75 ± 0.25 mm) and minimum inhibition in case of B. subtilis with 16 ± 1.5 mm. S. itriditicus (28.7.0 ± 0.5 mm) showed good inhibition with FHPH of P. acidilactici NCIM5368 followed by E. coli and Y. enterocolitica. Minimum inhibition was found in case of B. subtilis and S. typhi when treated with E. faecium NCIM5335, P. acidilactici FD3 whereas P. acidilactici NCIM5368 exhibited minimum inhibition with S. aureus and B. cereus. This may perhaps be due to the resistance that may result probably from alteration of bacterial membrane composition, destruction of the bacteriocin by proteases or altered receptors (Martinez and De Martinis 2005).
Table 3.
Antimicrobial activity (zone of inhibition in mm) of fermented fresh water fish head protein hydrolysate
| Organisms | NCIM5335 | FD3 | NCIM5368 |
|---|---|---|---|
| Antibacterial properties (zone of inhibition in mm) | |||
| E. coli MTCC118 | 28.0 ± 2.75a | 29.5 ± 2.00 a | 26.2 ± 2.25 a |
| B. cereus F4810 | 18.7 ± 0.25 a | 18.7 ± 0.25 a | 14.0 ± 0.50b |
| B. subtilis MTCC441 | 17.2 ± 1.25 a | 16.0 ± 1.50 a | 16.0 ± 2.00 a |
| Y. enterocolitica MTCC859 | 27.2 ±1.25 a | 27.5 ± 1.00 a | 26.7 ± 0.75 a |
| L. monocytogenes Scott A | 29.7 ± 0.25 a | 20.7 ± 0.75b | 13.5 ± 1.00c |
| S. aureus FRZ722 | 28.2 ± 0.92a | 29.7 ± 0.25a | 13.5 ± 0.50b |
| S. typhi FB231 | 17.5 ± 0.50 a | 15.0 ± 0.50b | 17.5 ± 0.50 a |
| S. paratyphi FB247 | 25.5 ± 0.50 a | 23.2 ± 0.25b | 25.0 ± 1.00 a |
| S. itriditicus FB257 | 28.2 ± 0.50 a | 28.7 ± 1.00 a | 27.0 ± 1.50 a |
| M. leuteus | 25.5 ± 0.50 a | 19.7 ± 3.25b | 20.5 ± 3.00b |
| Antifungal activity (% of inhibition) | |||
| A. niger MTCC218 | NI | NI | NI |
| A. ochraceus MTCC4643 | 28.0 ± 1.00 a | 18.0 ± 0.50b | 15.2 ± 1.00c |
| A. flavus MTCC277 | NI | NI | NI |
| A oryzae MTCC262 | 29.0 ± 1.00 a | 19.5 ± 1.50b | 16.20 ± 1.00c |
| P. chrysogenum MTCC616 | 55.5 ± 2.00 a | 39.4 ± 1.00b | 40.0 ± 2.00b |
| F. oxysporum MTCC1755 | 21.6 ± 2.00 a | 22.8 ± 2.00 a | 20.5 ± 1.00 a |
NCIM5335 - Enterococcus faecium NCIM5335, FD3 - Pediococcus acidilactici FD3, NCIM5368 - Pediococcus acidilactici NCIM5368; NI: No inhibition
Values within the same row with the same superscripts are not singnificantly different (p > 0.05) (n = 3)
Determination of Minimum Inhibitory Concentration (MIC) of FWPH in bacteria
The FHPH obtained on fermentation with E. faecium NCIM5335 had better antibacterial spectrum and effective antibacterial activity against pathogen like L. monocytogenes (29.75 ± 0.2) and S. itriditicus (28.0 ± 1.25) and hence considered for further studies to find the MIC of extracted protein hydrolysate on fermentation with E. faecium NCIM5335.
MIC of FHPH obtained on fermentation with E. faecium was calculated for L. monocytogenes and S. itridicus which were 10 and 12 mg/ml, respectively. The antibacterial activity increased with increasing concentration of the FHPH and indicates that antibacterial activity is dose dependent. The peptides have ability to depolarize the cytoplasmic membrane and are usually effective against Gram-positive microorganisms and are well supported by L. moncytogenes. LAB bacteriocins have varied mechanisms to exert an antimicrobial effect, but generally target the cell envelope. The initial electrostatic attraction between the target cell membrane and the bacteriocin peptide is thought to be the driving force for subsequent events (Deegan et al. 2006). Anionic cell surface polymers like teichoic acid and lipoteichoic acid may be important in the initial interaction of cationic bacteriocins of Gram-positive bacteria (Jack et al. 1995). The exact mode of action of antimicrobial peptides is widely believed that the peptides interact with and disrupt the cytoplasmic membrane, leading to the dissolution of the proton motive force and leakage of essential molecules, resulting in cell death. Bacteriocins may possess a bactericidal or bacteriostatic mode of action on sensitive cells, this distinction being greatly influenced by several factors such as bacteriocin dose and degree of purification, physiological state of the indicator cells and experimental conditions (Cintas et al. 2001).
Scanning electron microscopy studies on bacterial cells
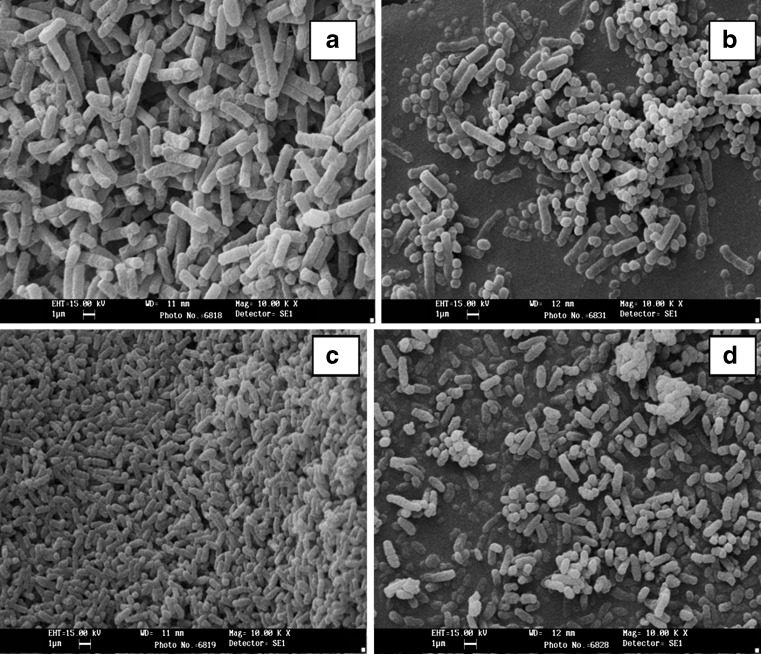
The FHPH effect observed under microscope revealed strong inhibition on cell growth. The cells of the bacteria appeared shriveled with degenerative size compared to untreated sample (Fig. 1). The inhibition caused may be due to some peptides. Moreover, bacteriocins are part of antimicrobial peptides and are widely believed that the carnage mechanism of these peptides on bacteria involves an interaction with the cytoplasmic membrane. Both groups act on sensitive cells by targeting either the inner membrane by pore formation or an intracellular target using enzymatic activity such as DNAse or RNAse (Abee 1995). Animal-derived antimicrobial peptides exhibit inhibitory effect against wide range of microorganisms than those produced by bacteria, as they are not affected by antibiotic- resistance mechanisms like other antibiotics (Rydlo et al. 2006).
Fig. 1.
Scanning electron microscopy of bacterial cells treated with protein hydrolysates. (E faecium NCIM5335). (a healthy cells of L. monocytogenes, b treated cells and c healthy cells of S. itriditicus, d treated cells)
Antifungal activity of FHPH
Antifungal susceptibility was observed in all the three protein hydrolysate extracted (Table 3). The FHPH extract fermentation with E. faecium NCIM5335 was found to be fairly efficient compared to P. acidilactici FD3 and P. acidilactici NCIM5368. The FHPH of NCIM5335 was effective against Penicillium compared to A. oryzae and A. ochareus. The delay in sporulation expressed as % of inhibition by FHPH of NCIM5335 against Penicillium, A. ochraceus and A. oryzae were 55 %, 28 %, and 29 %, respectively (Table 3), whereas A. niger and A. flavus were found to be resistant. Atanossova et al. (2003) reported to have characterized an antimicrobial proteinaceous compound from Lactobacillus paracasei sub sp. paracasei M3, with broad antibacterial activity and with fungistatic effects against 4 of the 26 tested yeast species. In contrast with the vast literature on bacteriocins, only few reports exist on antifungal peptides of LAB (Roy et al. 1996) and our results is one of the few to report the same.
Minimum inhibitory concentration of FHPH in fungi
The minimum inhibitory concentration of the protein hydrolysate against A. ochraceus was 60 mg/ml and 96 mg/ml for Penicillium. Likewise our results also suggest that the NCIM5335 extracts exhibited antifungal activity against A. ochraceus and Penicillium. Delayed growth and sporulation in case of Penicillium was observed. Different mechanisms of action have been proposed for peptides such as alteration of enzymatic activity, inhibition of spore germination and inactivation of anionic carriers (Martinez and De Martinis 2006). The FHPH did not exhibit antifungal activity against A. niger and A. flavus which could be due to influence of the proteolytic enzymes produced by these fungi in the hydrolysate (Batish et al. 1989). Possible explanations for these observations include synergistic effects between this peptide and host derived antifungal factors, such as endogenous antimicrobial proteins/peptides and reactive oxygen intermediates which has brought about inhibitory effect in case of fish epidermis (Claire Hellio et al. 2002).
Scanning electron microscopy studies of fungal cells
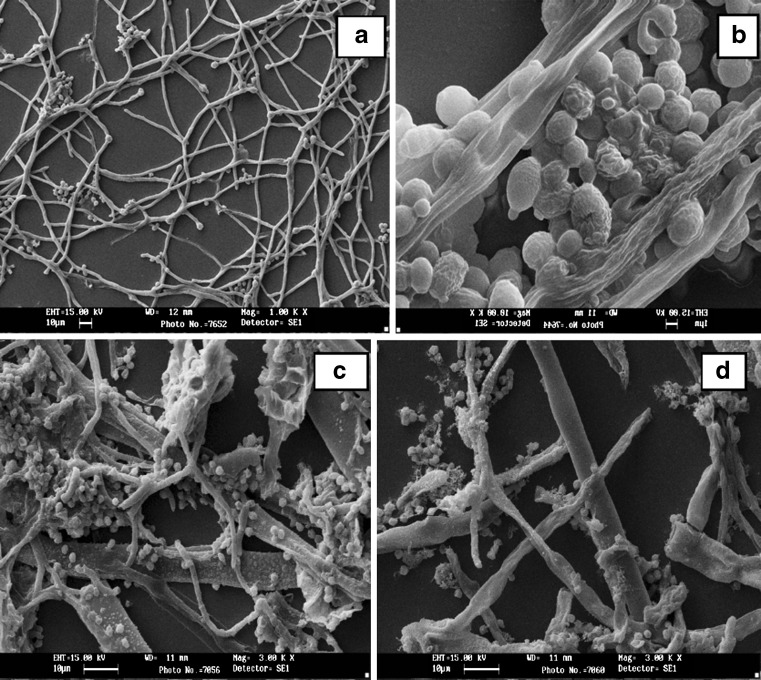
The SEM pictures of A. ochraceus and Penicillium sp exhibited alteration of the hyphal morphology when treated with NCIM5368 samples. The mycelium of untreated (control) A. ochraceus was illustrious with free and linearly shaped hyphae with anomalous branching (Fig. 2). But in case of FHPH treated fungi, notching of the terminal hyphae, shriveled hyphae, severely collapsed, cell surface depressions and squashed mycelium were observed (Fig. 2). This may be due to interferences of cell wall synthesis, which cause morphological changes and inhibit growth. Such modifications may be related to effect of peptides as enzymatic reactions regulate cell wall synthesis. This could be due to the loss of integrity of the cell wall and as a result, plasma membrane permeability might be affected, which explain changes in morphology with orientation to size and shape of the internal organelles. Also the antifungal activity involving cytoplasmic granulation, cytoplasmic membrane rupture and inactivation of intracellular and extracellular enzymes could have taken place. These biological events could take place separately or concomitantly, culminating in mycelial germination inhibition. However, it is likely that a careful reevaluation of other ‘bacteriocins’ might reveal further antifungal activities.
Fig. 2.
Scanning electron microscopy of hyphae treated with protein hydrolysates. (a healthy hyphae of A.ochraceus, b treated organisms with PH of E faecium NCIM5335, c healthy Penicillium and d treated)
Conclusions
The study collates information regarding oil rich in PUFA and peptidic hydrolysates with antioxidant and antimicrobial properties isolated from FWFH. Antioxidant and antimicrobial proteins isolated from fish head sources may be used as functional ingredient in food formulations to impart human health benefits and/or improve the shelf life of foods. Furthermore, fermented protein hydrolysate exhibited antimicrobial effects on various types of bacteria and finds its application in food preservation, food supplements and pharmaceutical industry.
Acknowledgements
Authors thank Department of Biotechnology (DBT), Govt. of India for partial funding of this work through Grant ##BT/PR 9474/AAQ/03/345/2007. Authors place on record their thanks to Director, CFTRI for encouragement and permission to publish the work.
References
- Abee T. Pore–forming bacteriocins of gram positive bacteria and self- protection mechanism of producer organisms. FEMS Microbiol Lett. 1995;129:1–10. doi: 10.1111/j.1574-6968.1995.tb07548.x. [DOI] [PubMed] [Google Scholar]
- Abee T, Krockel L, Hill C. Bacteriocins: modes of action and potentials in food preservation and control of food poisoning. Int J Food Microbial. 1995;28:168–185. doi: 10.1016/0168-1605(95)00055-0. [DOI] [PubMed] [Google Scholar]
- Ahmed J, Mahendrakar NS. Autolysis and rancidity development in fish viscera during fermentation. Biores Technol. 1996;58:247–251. doi: 10.1016/S0960-8524(96)00085-5. [DOI] [Google Scholar]
- Ajitha S, Sridhar M, Sridhar N, Singh ISB, Varghese V. Probiotic effects of lactic acid bacteria against Vibirio alginolyticus in penaeus (Fenneropenaeus) indicus (H. Milne Edwards) Asian Fish Sci. 2004;17:71–80. [Google Scholar]
- Amit KR, Bhaskar N, Halami PM, Indirani K, Suresh PV, Mahendrakar NS. Characterisation and application of a native lactic acid bacterium isolated from tannery fleshings for the fermentative bioconversion of tannery fleshing. Appl Microbiol Biotechnol. 2009;83:757–766. doi: 10.1007/s00253-009-1970-3. [DOI] [PubMed] [Google Scholar]
- Amit KR, Jini R, Swapna HC, Sachindra NM, Bhaskar N, Baskaran V. Application of native lactic acid bacteria (LAB) for fermentative recovery of lipids and proteins from fish processing wastes : Bioactivities of fermentation products. J Aquat Food Prod Tech. 2011;20:32–44. doi: 10.1080/10498850.2010.528174. [DOI] [Google Scholar]
- Official methods of analysis. 17. Washington, DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Atanossova M, Choiset Y, Dalgalarrondo M, Chobert JM, Dousset X, Ivanova I, Haertle T. Isolation and partial biochemical characterization of a proteinaceous antibacterial and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int J Food Microbiol. 2003;87:63–73. doi: 10.1016/S0168-1605(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Batish VK, Grover S, Lal R. Screening lactic starter cultures for antifungal activity. Cult Dairy Prod J. 1989;24:21–25. [Google Scholar]
- Bijinu B, Binod P, Amit KR, Suresh PV, Mahendrakar NS, Bhaskar N. In vitro antioxidant and antibacterial properties of delimed tannery fleshings: comparison of acid hydrolysis and fermentation method. Biodegrad. 2011;22:287–295. doi: 10.1007/s10532-010-9398-0. [DOI] [PubMed] [Google Scholar]
- Cintas LM, Herranz C, Hernández PE, Casaus MP, Nes LF. Review: bacteriocins of lactic acid bacteria. Food Sci Tech Int. 2001;7:281–305. doi: 10.1177/108201301772660538. [DOI] [Google Scholar]
- Claire Hellio MP, Claude B, Nathalie B, Yves LG. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int J Antimicrob Agents. 2002;20:214–219. doi: 10.1016/S0924-8579(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Deegan LH, Cotter PD, Hill C, Ross P. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J. 2006;16:1058–1071. doi: 10.1016/j.idairyj.2005.10.026. [DOI] [Google Scholar]
- Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998;11:57–80. doi: 10.1128/cmr.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2010) Year book of fishery statistics—Latest summary of tables (Available at http://www.fao.org/fishery/statistics/en;last accessed on May 29, 2010)
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. Eur J Food Sci Tech. 2006;108:766–777. [Google Scholar]
- Gediminas BA, Olga KV, Jurgita K. Food-Protein enzymatic hydrolysates posseses both antimicrobial and immunostimulatory activities: a cause and effect theory of biofunctionality. FEMS Immunol Med Microbiol. 2006;46:131–138. doi: 10.1111/j.1574-695X.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- Jack RW, Tagg JR, Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jini R, Swapna HC, Amit KR, Vrinda R, Halami PM, Sachindra NM, Bhaskar N. Isolation and characterization of potential lactic acid bacteria (LAB) from freshwater fish processing wastes for application in fermentative utilisation of fish processing waste. Braz J Microbiol. 2011;42:1516–1525. doi: 10.1590/S1517-83822011000400039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Shimazaki K, Higuchi H, Nagahata H. Antibacterial activity of bovine lactoferrin hydrolysate against mastitis pathogens and its effect on superoxide roduction of bovine neutrophils. Zoo Pub Health. 2007;54:160–164. doi: 10.1111/j.1863-2378.2007.01031.x. [DOI] [PubMed] [Google Scholar]
- Martinez RCR, De Martinis ECP. Antilisterial activity of a crude preparation of Lactobacillussakei 1 bacteriocin and its lack of influence on Listeria monocytogenes haemolytic activity. Food Cont. 2005;16:429–433. doi: 10.1016/j.foodcont.2004.05.002. [DOI] [Google Scholar]
- Martinez RCR, De Martinis ECP. Effect of Leuconosoc mesenteroides 11 bacteriocin in the multiplication control of Listeria monocytogenes. Ciênc Tecnol Aliment. 2006;26:52–55. doi: 10.1590/S0101-20612006000100009. [DOI] [Google Scholar]
- Nayak J, Nair PGV, Mathew S, Ammu K. A study on the intestinal lipase of Indian major carp Labeo rohita. Asian Fish Sci. 2004;17:333–339. [Google Scholar]
- Rajaram D, Nazeer RA. Antioxidant properties of protein hydrolysates obtained from marine fishes Lepturacanthus savala and Sphyraena barracuda. Int J Biotechnol Biochem. 2010;6:435–444. [Google Scholar]
- Roy U, Batist VK, Grover S, Neelakantan S. Production of antifungal substance by Lactococcus lactis subsp. lactis CHD-28.3. Int J Food Microbiol. 1996;32:27–34. doi: 10.1016/0168-1605(96)01101-4. [DOI] [PubMed] [Google Scholar]
- Rydlo T, Miltz J, Mor A. Eukaryotic antimicrobial peptides: promises and premises in food safety. J Food Sci. 2006;71:125–135. doi: 10.1111/j.1750-3841.2006.00175.x. [DOI] [Google Scholar]
- Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003;82:593–597. doi: 10.1016/S0308-8146(03)00015-3. [DOI] [Google Scholar]
- Singh G, Maurya S, Catalan C, Lampasoma MP. Chemical, antifungal, antioxidative studies of Ajwain oil and its acetone extract. J Agric Food Chem. 2004;52:3292–3296. doi: 10.1021/jf035211c. [DOI] [PubMed] [Google Scholar]
- Statistica for Windows. Tulsa: Statsoft Inc.; 1999. [Google Scholar]
- Swapna HC, Amit KR, Bhaskar N, Sachindra NM. Lipid classes and fatty acid profile of selected Indian fresh water fishes. J Food Sci Technol. 2010;47:394–400. doi: 10.1007/s13197-010-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongson C, Davidson PM, Mahakarrchanakul W, Weiss J. Antimicrobial activity of ultrasound- assisted solvent-extracted spices. Lett Appl Microbiol. 2004;39:401–406. doi: 10.1111/j.1472-765X.2004.01605.x. [DOI] [PubMed] [Google Scholar]
- Turchini GM, Torstensen BE, Ng WK. Fish oil replacement in finfish nutrition. Rev Aquaculture. 2009;1:10–57. doi: 10.1111/j.1753-5131.2008.01001.x. [DOI] [Google Scholar]