Fig. 1.
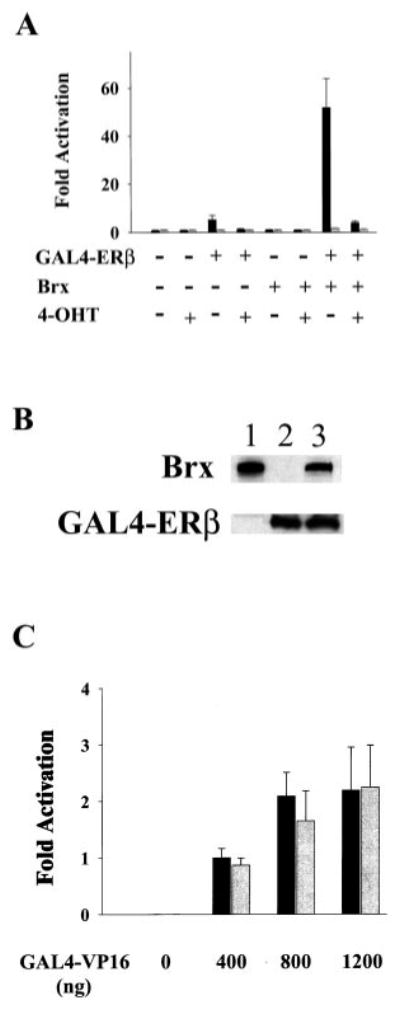
A, Brx co-expression augments ligand-dependent activation of a G4E1b-luciferase reporter plasmid by GAL4-ERβ. Augmentation of ERβ-mediated activation of G4E1b-luciferase by Brx co-expression is inhibited by the addition of 4-hydroxy tamoxifen. Ishikawa cells were transfected with 1 μg of G4E1b-luciferase and expression vectors for GAL4-ERβ3 (500 ng) and Brx (1 μg) or the respective amounts of empty vectors as control. Estradiol (10 nm, solid bars) or vehicle control (gray bars), and vehicle control or 4-hydroxytamoxifen (40 nm) was added to the cells as indicated. Cells were harvested after 20 h. Luciferase values represent -fold activation (mean) over control (RSV-0 with G4E1b-luciferase) in the absence of ligand, under each condition from three experiments performed in triplicate. Error bars represent standard deviations. B, Brx did not affect receptor expression levels. COS-7 cells were transfected with expression vectors for Brx (lane 1), GAL4-ERβ (lane 2), or both (lane 3). Brx and GAL4-ERβ were detected in lysates of transfected cells by Western blotting. Plasmid amounts were held constant by addition of empty expression vectors. C, Brx does not augment the activity of a chimeric GAL4-VP16 transcription factor. Ishikawa cells were transfected with 1 μg of G4E1b-luciferase reporter plasmid and expression vectors for GAL4-VP16 (0, 400, 800, or 1200 ng, as indicated) and Brx (1 μg, gray bars) or the same amounts of empty expression vectors (solid bars). Cells were harvested and assayed for luciferase activity after 20 h. Luciferase values represent means of-fold activation (with activation given by 400 ng of GAL4-VP16 arbitrarily assigned a value of 1.0) from two experiments performed in duplicate. Error bars represent standard deviations.
