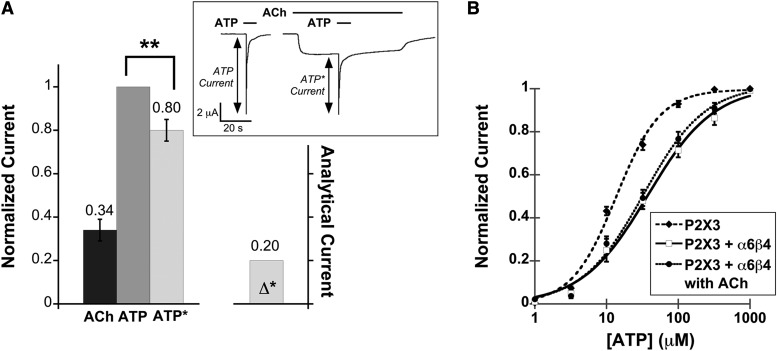
Fig. 4.
Functional interaction between the α6β4 nAChR and the homomeric P2X3 receptor. (A) Mean, normalized current ± S.E.M. is shown for the peak of agonist-induced currents measured from P2X3–α6β4 oocytes (n = 12) upon application of ACh (100 μM), ATP (100 μM), and ATP with ACh preapplication (ATP*). Mean current amplitudes ± S.E.M. are 2.57 ± 0.50 μA for ACh, 7.29 ± 0.65 μA for ATP, and 6.4 ± 0.75 μA for ATP*. All measurements were normalized to the ATP current of the same cell and then averaged. Δ* is the mean difference between IATP and IATP*. **P < 0.005. The insert shows the protocol used for probing cross inhibition between the α6β4 nAChR and the fast-desensitizing P2X receptor. ATP was applied alone or after a preapplication of ACh. For both IATP and IATP*, at least three agonist-induced currents were averaged from the same cell. The resulting IATP and IATP* currents were then compared with determine cross inhibition. (B) ATP dose-response curves for P2X3 oocytes (EC50 13.6 ± 1.3 μM, Hill constant 1.4 ± 0.16, n = 12), P2X3–α6β4 oocytes in the absence of ACh (EC50 37.8 ± 6.1 μM, Hill constant 0.94 ± 0.11, n = 14), and P2X3–α6β4 oocytes in the presence of 100 μM ACh (EC50 32.8 ± 5.0 μM, Hill constant 1.0 ± 0.12, n = 11). The fitted curves show that the P2X3 cells were less sensitive to ATP when α6β4 was coexpressed, regardless of nAChR activation by ACh.