Abstract
Rationale:
Pediatric obstructive sleep apnea (OSA) is common, and a delay in diagnosis can lead to significant morbidity. Polysomnography (PSG) is the gold standard for the diagnosis of OSA. However, difficulty accessing PSG due to the relative shortage of sleep centers with pediatric expertise can lead to a delay in the diagnosis and management of OSA.
Objectives:
To assess the utility of Mallampati score (sitting and supine) in predicting the presence and severity of OSA in children.
Methods:
A retrospective study of 158 children from a single pediatric sleep center. All patients had a PSG and a physical examination documenting Mallampati score. The Mallampati score, tonsillar size, age, sex, and apnea hypopnea index (AHI) were analyzed. Odds ratio of having pediatric OSA (AHI > 1) with increase in Mallampati score and tonsillar size were calculated.
Measurements and Main Results:
A significant correlation was found between Mallampati score, tonsillar size, and AHI. For every point increase in the Mallampati score, the odds ratio of having OSA increased by more than 6-fold. For every point increase in tonsillar size, the odds ratio of having OSA increased by more than 2-fold.
Conclusions:
Mallampati score and tonsillar size are independent predictors of OSA. Oral examination including Mallampati score and tonsillar size should be considered when evaluating a patient for OSA. They can be used to prioritize children who may need PSG.
Citation:
Kumar HVM, Schroeder JW Jr, Gang Z, Sheldon SH. Mallampati score and pediatric obstructive sleep apnea. J Clin Sleep Med 2014;10(9):985-990.
Keywords: pediatric obstructive sleep apnea, Mallampati score, tonsillar size
Pediatric obstructive sleep apnea (OSA) is a common condition and is currently estimated to affect between 1% and 3% of 2- to 8-year old children.1–3 The potential consequences of OSA in children include behavioral disturbances, learning deficits, pulmonary hypertension, systemic hypertension, and compromised somatic growth.1,4–9 The long-term effects of childhood OSA remain incompletely understood. Several studies have tried to determine the utility of predicting pediatric obstructive sleep apnea using questionnaires, history, and physical exam.10,11 Most have reported a poor sensitivity, specificity, positive predictive value, and negative predictive value.12–14 There is currently no universally accepted tool that uses history and clinical exam alone to diagnose pediatric OSA.15 None of these have included Mallampati score in the clinical exam. The American Academy of Pediatrics (AAP) clinical practice guidelines from 2002 and 2012 consider PSG the gold standard for establishing the presence and severity of OSA in children, due to the difficulty in differentiating pediatric OSA from primary snoring by means of history and physical exam alone.16,17 The clinical practice guidelines from the American Academy of Otolaryngology Head and Neck Surgery do advocate PSG, but they are not required for every patient who undergoes tonsillectomy and adenoidectomy (T & A).18 Obtaining PSG on every patient prior to T & A may not be feasible because pediatric PSG are not universally available, primarily due to the scarcity of dedicated pediatric sleep centers. In addition, pediatric PSG are expensive and universal PSG may not be the most efficient and effective use of health care dollars.
BRIEF SUMMARY
Current Knowledge/Study Rationale: History and physical exam are not sufficient to make the diagnosis of pediatric obstructive sleep apnea. Polysomnography (PSG) is the gold standard used to confirm this diagnosis and is therefore recommended in certain clinical situations. However, PSG is an expensive test and access to pediatric sleep labs is limited.
Study Impact: Mallampati score and tonsillar size are independent predictors for the presence of and the severity of pediatric OSA and may be used to help prioritize sleep studies for earlier diagnosis and management of pediatric OSA.
Mallampati scoring is a simple, noninvasive, inexpensive technique that involves visualization of the oropharynx. It is easy to learn and does not require any special equipment or setting. It has been used for more than two decades to assess the ease of intubation in anesthesiology.19–22 The American Academy of Sleep Medicine and Dutch Central Accompagnement Organization (CBO) both state that the Mallampati score has additional value in diagnosing OSA in adults.23 There are several adult studies with mixed results regarding the utility of Mallampati score in predicting OSA.24–31 There have been no studies in the pediatric population looking at the clinical use of Mallampati score to predict obstructive sleep apnea and its severity.
Objectives
The primary objective of this retrospective study was to evaluate if the Mallampati score can predict pediatric OSA. If the Mallampati score was found to predict pediatric OSA, then the second objective was to evaluate if the Mallampati score could also predict the severity of pediatric OSA.
MATERIALS AND METHODS
Study Population
This retrospective study was performed at Ann & Robert H. Lurie Children's Hospital of Chicago (formerly Children's Memorial Hospital, Chicago, Illinois), and it was approved by the institutional review board. All patients between age ≥ 3 years and < 18 years with a documented Mallampati score at the time of physical examination and who had a polysomnogram were included in this study. Relevant medical data were extracted from the electronic medical record. Patients were excluded from the study if they had chronic lung disease, craniofacial malformations, trisomy 21, history of tonsillectomy and/or adenoidectomy, developmental delay, prematurity < 37 weeks of gestation, or active tonsillitis/pharyngitis at the time of physical exam. Two hundred forty-one charts were screened retrospectively of patients who had a sleep study and Mallampati score documented on physical exam; 83 were excluded, leaving a total of 158 in the study. The excluded patients included 25 patients with Down syndrome, 56 patients post-adenotonsillectomy, 1 patient with achondroplasia, and 1 patient with Crouzon syndrome.
Measurements
Age, sex, Mallampati score (taken both when the patient was sitting and supine), tonsillar size, apnea hypopnea index (AHI), and the patient's diagnosis at the time of the exam were extracted from the electronic medical record.
Mallampati Score
The Mallampati score was obtained in children who were cooperative and allowed visualization of their oropharynx. The Mallampati score was obtained in both the sitting and in the supine position when possible. Patients were allowed to emit sound when the mouth was open. Scoring depends on visibility of anatomical structures in the oropharynx. The anatomical structures include faucial/tonsillar pillars (arches in front and behind the tonsils), base of the uvula, and soft palate. It is important to note that tonsillar size does not affect the Mallampati score (Figure 1).
Figure 1. Mallampati score.
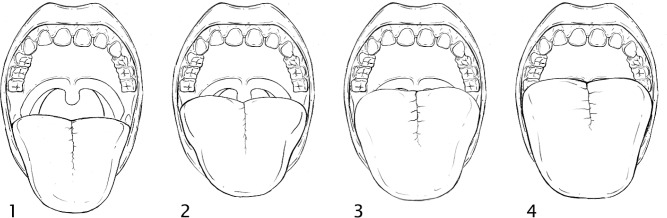
Class 1: Faucial/tonsillar pillars, uvula and soft palate are all visible. Class 2: Partial visibility of the faucial/tonsillar pillars, uvula and soft palate. Class 3: Base of the uvula, soft and hard palate visible. Class 4: Only hard palate is visible.
Tonsil Size
Tonsillar size was scored when the patient had an open mouth and tongue in neutral position. It was okay to emit sound during this process. A tongue depressor was used if the patient had a higher Mallampati score blocking the visualization of the tonsils. Tonsil size was graded from 0 to 4, depending on the size of the tonsils. Mallampati score does not affect tonsil size score (Figure 2).
Figure 2. Tonsillar size.
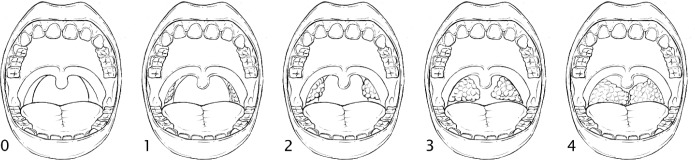
Grade 0: No tonsil tissue present. Grade 1: Tonsils hidden within the faucial/tonsillar pillars. Grade 2: Tonsils extending to the pillars but not beyond them. Grade 3: Tonsils extending beyond the faucial/tonsillar pillars but not to the midline. Grade 4: Tonsils extending to the midline and may be touching each other.
Polysomnography
All patients included in the study had a comprehensive polysomnogram performed at a pediatric sleep lab consisting of continuous nocturnal monitoring of EEG (bipolar transcoronal and parasagittal electrode array) using the International 10-20 System of electrode placement; EOG (left outer canthus and right outer canthus); chin muscle EMG (mostly measures genioglossus activity); left and right anterior tibialis EMG; lead II EKG; instantaneous heart rate (RR interval by FFT analysis); nasal/oral airflow by capnography and pressure transduction; EtCO2 trend by capnometry; chest and abdominal wall respiratory movement by respiratory inductance plethysmography (RIP) technology (measures changes in circumference); body position; upper respiratory tract sound by sonography; and oxygen saturation by pulse oximetry. Continuous pulse volume was measured using finger plethysmography. Chest and abdominal phase angle analysis was also conducted. The record was scored and evaluated according to standards set by the American Academy of Sleep Medicine.32 Analysis was conducted in 30-s epochs. All epochs of the recording were analyzed.
Comprehensive evaluation of sleep-related respiratory status was conducted. The number of occlusive (apneas) and partially occlusive (hypopneas) respiratory events was provided. Significant central apneas and/or hypopneas and/or periodic breathing were reported in the impression. The apnea index (AI) represents the total number of apneas divided by the total sleep time. The apnea-hypopnea index (AHI) represents the total number of apneas plus hypopneas per hour of sleep. The AI and AHI did not include central apneas. Baseline and nadir SaO2 were reported. Baseline and maximum EtCO2 were reported. All polysomnograms were scored by a qualified sleep technologist and interpreted by a board certified sleep physician. Patients with AHI > 1 were considered to have obstructive sleep apnea.
Statistical Methods
The Mallampati score and the tonsil size in children with OSA were compared to the values in children without OSA. In the children with OSA, the severity of the OSA as determined by the AHI was correlated to the Mallampati score and tonsil size. Fisher exact test was used to analyze the association between Mallampati score, tonsillar size, and OSA. The odds ratio of having OSA for every point increase in Mallampati score (sitting and supine) and tonsillar size was calculated. The Pearson χ2 test was used to examine the association between sex and age of the patient and pediatric OSA. A logistic regression model was created with OSA as the outcome and tonsillar size, Mallampati score sitting, and Mallampati score supine as variables to determine if one was superior.
RESULTS
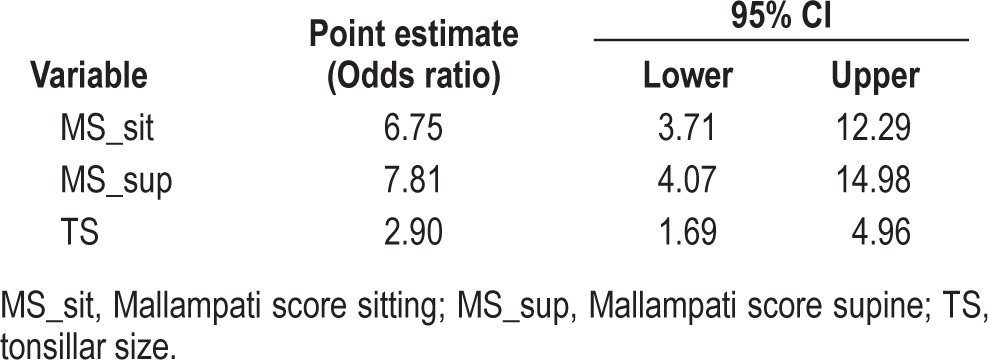
Of 158 patients included in the study (Table 1), 90 patients had OSA (AHI ≥ 1) and 68 patients did not have OSA (AHI < 1). Mallampati score sitting, Mallampati score supine, and tonsillar size individually demonstrated a strong association with OSA by Fisher exact test (Table 2). The odds ratio for having OSA increased independently as the Mallampati score (sitting and supine) and tonsillar size increased. For every point increase in the Mallampati score-sitting, the odds ratio of having OSA increased by more than 6-fold (odds ratio for 1-point increase was 6.75; 95% confidence interval: 3.71–12.29; p < 0.01). For every point increase in the Mallampati score-supine, the odds ratio of having OSA increased by more than 7-fold (odds ratio for 1-point increase was 7.81; 95% CI: 4.07–14.98; p < 0.01). For every point increase in tonsillar size, the odds ratio of having OSA increased by more than 2-fold, odds ratio for 1-point increase in tonsillar size was 2.90; 95% confidence interval: 1.69–4.15; p < 0.01 (Table 3). The Pearson χ2 test was used to examine the association between sex and age of the patient and pediatric OSA, and there was no significant association.
Table 1.
Characteristics of the 158 patients included in the study.
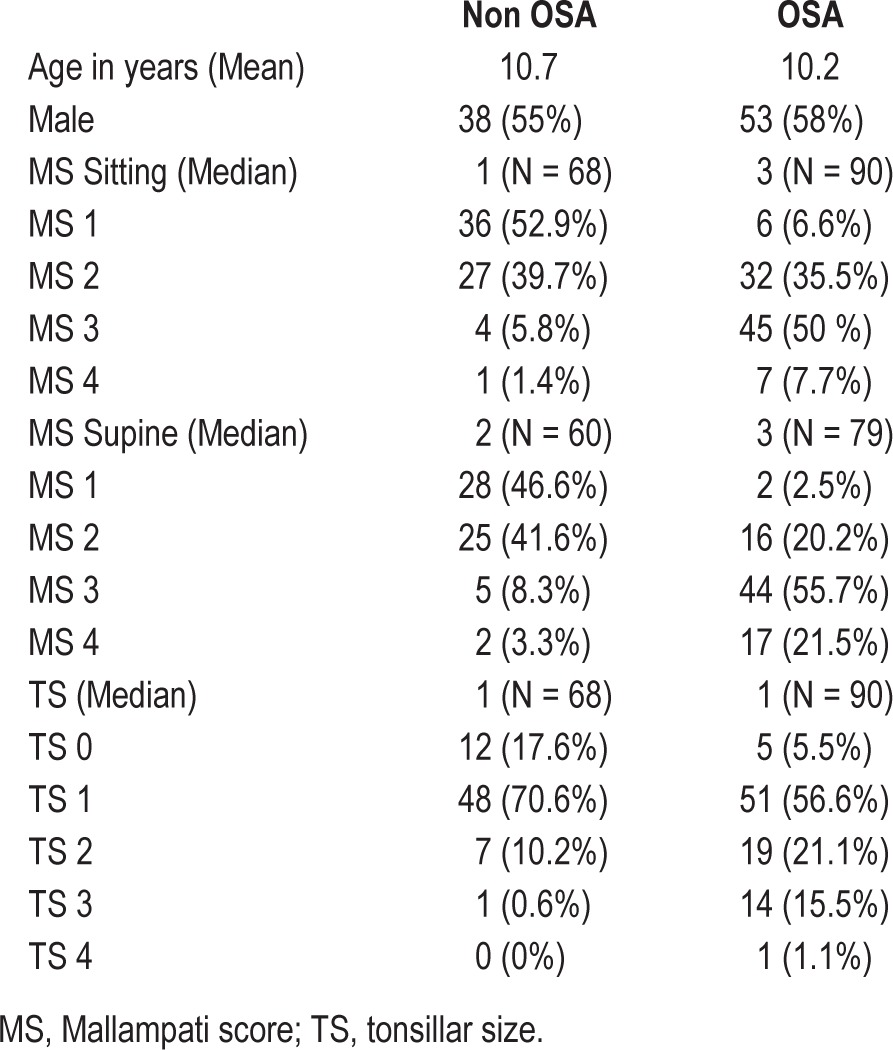
Table 2.
Pediatric OSA and its association with Mallampati score (sitting and supine) and tonsillar size.

Table 3.
Odds ratio of having OSA for Mallampati score
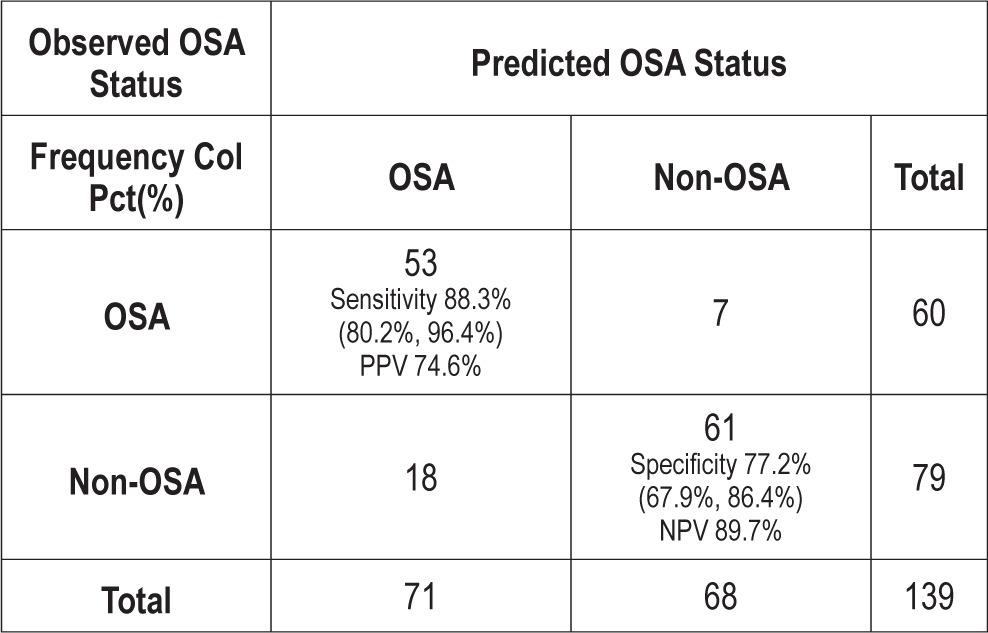
A logistic regression model with OSA as the outcome and tonsillar size, Mallampati score sitting, and Mallampati score supine was created. Backward selection process was performed, and the Mallampati score supine was the only predictor that stayed in the model—demonstrating that Mallampati score supine is superior to Mallampati score sitting and tonsillar size in predicting OSA. In this model, Mallampati score supine was able to predict OSA with a sensitivity of 89.7% and specificity of 74.6% (Table 4).
Table 4.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of Mallampati score supine in predicting OSA.
When considered individually, Mallampati score supine, Mallampati score sitting, and tonsillar size have a strong linear correlation with AHI and hence OSA (Table 5 and Figure 3). Receiver operating characteristic (ROC) curve comparison was performed for Mallampati score supine, Mallampati score sitting, and tonsillar size. The area under the curve was greatest for Mallampati score supine, indicating that it is superior to Mallampati score sitting and tonsillar size (Figure 4).
Table 5.
Pearson correlation between Mallampati score (sitting and supine) and tonsillar size with apnea hypopnea index (AHI) and OSA.
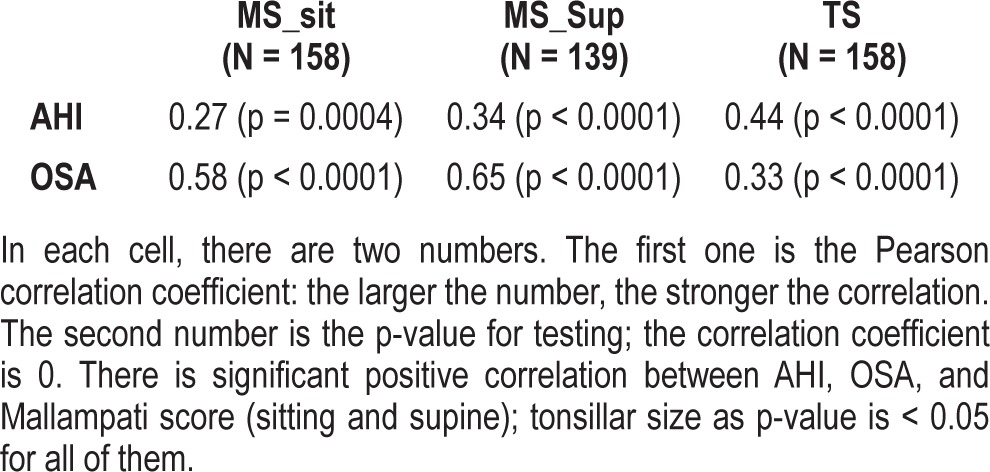
Figure 3. Box plot showing AHI vs. MS_sit, MS_sup, and tonsillar size.
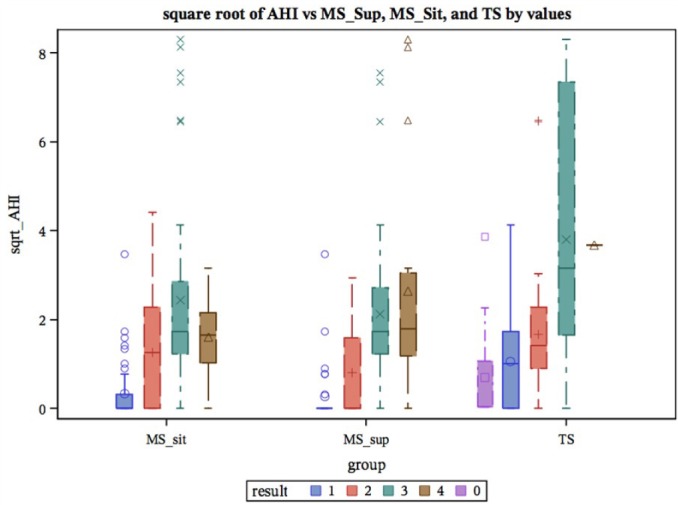
AHI, apnea hypopnea index; MS_sit (1-4), Mallampati score sitting, MS_ sup (1-4) Mallampati score supine; TS (0-4), tonsillar size
Figure 4. Receiver operating characteristic (ROC) Curve comparison for Mallampati score supine, Mallampati score sitting, and tonsillar size.
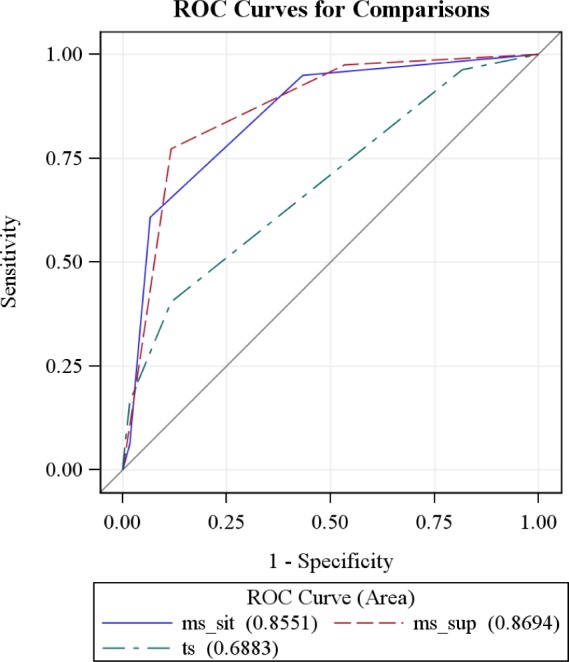
The ROC curves for the three models are compared in the same plot above. The area under curve (AUC) is largest for Mallampati score supine, indicating that it is a better predictor when compared to Mallampati score sitting and tonsillar size.
DISCUSSION
Pediatric obstructive sleep apnea can cause significant morbidity, and it affects a large number of children. A screening tool that can effectively and economically identify children with OSA would be ideal. Currently, no such widely accepted screening tool exists. Several studies have demonstrated that history and physical exam have an unacceptably low sensitivity and specificity when it comes to differentiating primary snoring from OSA.12–14 As a result, PSG remains the gold standard for diagnosing OSA.17 However, the lack of access to dedicated pediatric sleep labs can make this test expensive and impractical. To this end, this study evaluated the utility of Mallampati score and tonsillar size for determining the presence of OSA in children. We determined that Mallampati score and tonsillar size were independent predictors for determining the presence of pediatric OSA, and they also correlate with its severity. It is important to note that, in this study, a patient was considered to have OSA if the AHI was greater than 1.
Mallampati Score
Mallampati score assessment (sitting and supine) are noninvasive measurements that are easy to learn and can be performed in very little time in any physician's office. On average, the odds of having OSA increases by more than 6-fold for every point increase in Mallampati score. The AHI (severity of OSA) also has a positive correlation with the increase in Mallampati score. Adult studies have shown mixed results regarding correlation between OSA and Mallampati score.23,25 In children, a high Mallampati score may be more predictive of the presence of OSA than in adults because the main cause of the obstruction is pharyngeal. Because the obstruction is often multifaceted, surgical procedures alone are not sufficient to cure the obstruction in the adult population. It is important to note that Mallampati score is independent of tonsil size. It is unclear if a high Mallampati score is associated with a higher rate of persistent OSA in children after T & A. This is an area that requires further study.
Tonsillar Size
Tonsillar size measurement is also noninvasive, is easy to learn, and can be performed in any clinical setting. On average, the odds of having OSA increase by more than 2-fold for every point increase in tonsillar size. Earlier studies have shown mixed results regarding the correlation of tonsillar size on physical exam and OSA.33–35 In our study, there was a positive correlation between tonsillar size and pediatric OSA. Disproportionately large tonsils, with or without disproportionately small oropharyngeal cavity, can lead to obstruction, and contribute to presence of OSA and its severity. Tonsil position may be more important that tonsil size. Exophytic tonsils may cause more obstruction in sleep than the endophytic tonsils, and the mixed results in various studies could be secondary to this. There are studies that have shown that true tonsil size using magnetic resonance imaging36 and post-surgical removal volume measurements37 have a positive correlation with OSA. This emphasizes the idea that in vivo tonsil position may play a more significant role
Mallampati score (sitting and supine) and tonsillar size were independent predictors of OSA and its severity, but the Mallampati score supine was superior to the other two. This is possibly explained by the fact that in the supine position (which mimics the sleep position), the tongue and the surrounding soft tissue structures can fall back and cause further narrowing of the oropharynx. The Mallampati score supine was able to predict OSA with a sensitivity of 89.7% and specificity of 74.6% compared to tonsillar size, which had a sensitivity of 70.5% and specificity of 60%.
This study has limitations. Because it is a retrospective study conducted in one urban tertiary care children's hospital, the results may not be generalized. However, the children in the study represented a diverse population. Children were seen in a sleep clinic based on a referral for a suspected sleep related problem, which may have introduced a selection bias; to minimize the selection bias, children without snoring were also included in the study. Within-rater variation is not available for this study, and it is a limitation.
We suggest that, based on findings from this study, Mallampati score can be used to prioritize patients who need to get a sleep study. Patients with increased scores can be prioritized for a sleep study and/or a referral to sleep medicine physicians. This can lead to earlier diagnosis and more timely management of OSA. Delayed diagnosis of OSA not only leads to a poorer quality of life and neurologic and cardiopulmonary morbidities but also increases health care costs. Polysomnography is the gold standard for the diagnosis of pediatric OSA, and the Mallampati score is an additional tool to help identify patients at increased risk for pediatric OSA. Mallampati score and tonsil size scores, at this time, cannot supplant the need for a PSG. Further prospective studies are required to validate the findings of our retrospective study and also to assess the utility of Mallampati score as a simple diagnostic test for predicting pediatric obstructive sleep apnea. However, we believe, based on the results of this study, that both Mallampati score and tonsil size are very useful in accurately predicting both the presence of and severity of OSA in children.
CONCLUSION
Mallampati score and tonsil size are independent predictors for the presence of OSA in children. The Mallampati score taken in a supine position is a better predictor of the presence of OSA than Mallampati score in a sitting position and tonsil size. Larger tonsil size and higher Mallampati scores are associated with more severe OSA as defined by higher AHI scores. Mallampati score and tonsillar score along with a comprehensive sleep history seem to be promising for screening children at risk for OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. There was no drug used off-label or as an investigational drug for this study
REFERENCES
- 1.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68:360–6. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gislason T, Benediktsdottir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995;107:963–6. doi: 10.1378/chest.107.4.963. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 4.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring: evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139:165–71. doi: 10.1007/BF01377349. [DOI] [PubMed] [Google Scholar]
- 5.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 7.Shiomi T, Guilleminault C, Stoohs R, Schnittger I. Obstructed breathing in children during sleep monitored by echocardiography. Acta Paediatr. 1993;82:863–71. doi: 10.1111/j.1651-2227.1993.tb17629.x. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 9.Everett AD, Koch WC, Saulsbury FT. Failure to thrive due to obstructive sleep apnea. Clin Pediatr. 1987;26:90–2. doi: 10.1177/000992288702600206. [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 11.Schechter MS Section on Pediatric Pulmonology. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 12.Preutthipan A, Chantarojanasiri T, Suwanjutha S, Udomsubpayakul U. Can parents predict the severity of childhood obstructive sleep apnoea? Acta Paediatr. 2000;89:708–12. doi: 10.1080/080352500750044052. [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Otolaryngol Head Neck Surg. 2007;133:216–22. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 15.van Someren V, Burmester M, Alusi G, Lane R. Are sleep studies worth doing? Arch Dis Child. 2000;83:76–81. doi: 10.1136/adc.83.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farber JM. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;110:1255–7. doi: 10.1542/peds.110.6.1255-a. author reply 55-7. [DOI] [PubMed] [Google Scholar]
- 17.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 18.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: Polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145:S1–15. doi: 10.1177/0194599811409837. [DOI] [PubMed] [Google Scholar]
- 19.Mallampati SR. Clinical sign to predict difficult tracheal intubation (hypothesis) Can Anaesth Soc J. 1983;30:316–7. doi: 10.1007/BF03013818. [DOI] [PubMed] [Google Scholar]
- 20.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–34. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 21.Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–90. doi: 10.1111/j.1365-2044.1987.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 22.Benumof JL. Management of the difficult adult airway. With special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–110. doi: 10.1097/00000542-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 24.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–8. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 25.Bins S, Koster TD, de Heij AH, et al. No evidence for diagnostic value of Mallampati score in patients suspected of having obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 2011;145:199–203. doi: 10.1177/0194599811409302. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran SK, Kheterpal S, Consens F, et al. Derivation and validation of a simple perioperative sleep apnea prediction score. Anesth Analg. 2010;110:1007–15. doi: 10.1213/ANE.0b013e3181d489b0. [DOI] [PubMed] [Google Scholar]
- 27.Dahlqvist J, Klar J, Tiwari N, et al. A single-nucleotide deletion in the POMP 5' UTR causes a transcriptional switch and altered epidermal proteasome distribution in KLICK genodermatosis. Am J Hum Genet. 2010;86:596–603. doi: 10.1016/j.ajhg.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog M, Metz T, Schmidt A, et al. The prognostic value of simulated snoring in awake patients with suspected sleep-disordered breathing: introduction of a new technique of examination. Sleep. 2006;29:1456–62. doi: 10.1093/sleep/29.11.1456. [DOI] [PubMed] [Google Scholar]
- 29.Liistro G, Rombaux P, Belge C, Dury M, Aubert G, Rodenstein DO. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J. 2003;21:248–52. doi: 10.1183/09031936.03.00292403. [DOI] [PubMed] [Google Scholar]
- 30.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 31.Hukins C. Mallampati class is not useful in the clinical assessment of sleep clinic patients. J Clin Sleep Med. 2010;6:545–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 33.Certal V, Catumbela E, Winck JC, Azevedo I, Teixeira-Pinto A, Costa-Pereira A Clinical assessment of pediatric obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2012;122:2105–14. doi: 10.1002/lary.23465. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues MM, Dibbern RS, Goulart CW. Nasal obstruction and high Mallampati score as risk factors for obstructive sleep apnea. Braz J Otorhinolaryngol. 2010;76:596–9. doi: 10.1590/S1808-86942010000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolan J, Brietzke SE. Systematic review of pediatric tonsil size and polysomnogram-measured obstructive sleep apnea severity. Otolaryngol Head Neck Surg. 2011;144:844–50. doi: 10.1177/0194599811400683. [DOI] [PubMed] [Google Scholar]
- 36.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 37.Howard NS, Brietzke SE. Pediatric tonsil size: objective vs subjective measurements correlated to overnight polysomnogram. Otolaryngol Head Neck Surg. 2009;140:675–81. doi: 10.1016/j.otohns.2009.01.008. [DOI] [PubMed] [Google Scholar]


