Scheme 1.
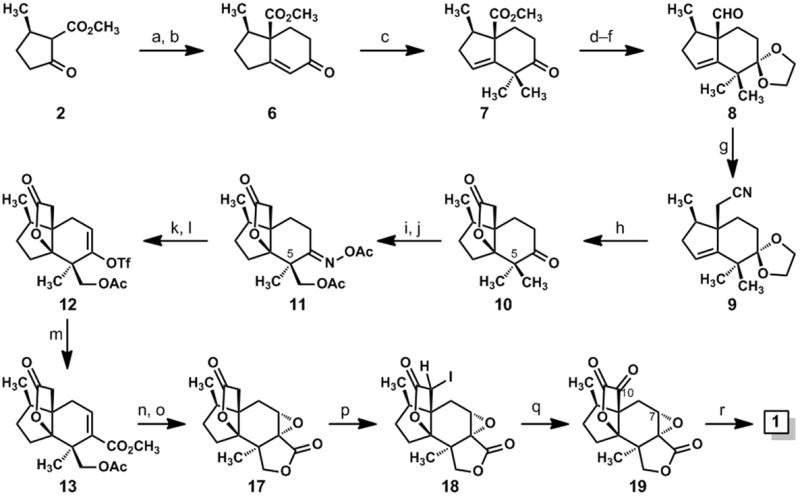
Synthesis of jiadifenolide: (a) methyl vinyl ketone (1.2 eq.), DBU (0.25 eq.), EtOH, rt, 97%; (b)p-TsOH (0.8 eq.), benzene, reflux, 85%; (c) CH3I (6 eq.), KOt-Bu (3.1 eq.), t-BuOH, rt, 91%; (d) ethylene glycol (6 eq.), p-TsOH (0.1 eq.), benzene, reflux; (e) DIBAL-H (3.1 eq.), CH2Cl2, –78 °C→ 0 °C, 77% (over two steps); (f) (COCl)2 (1.5 eq.), DMSO (3 eq.); then Et3N (5 eq.), CH2Cl2, –78 °C → rt, 90%; (g) TosMIC (1.7 eq.), KOt-Bu (2.6 eq.), THF, –50 °C, then CH3OH, 65 °C, 90%; (h) H2SO4, CH3OH, 100 °C, 73%; (i) NH2OH•HCl (1.5 eq.), pyridine, 80 °C, 91%; (j) Pd(OAc)2 (0.05 eq.), PhI(OAc)2 (1.5 eq.), 1:1 Ac2O/AcOH, 100 °C, 12 h, 22% of 11; (k) Fe (10 eq.), AcOH (cat.), Me3SiCl (cat.), THF, rt, 89%; (l) KN(SiMe3)2 (1.1 eq.), Comins reagent (1.2 eq.), THF, –78 °C; (m) Pd(OAc)2 (0.1 eq.), Ph3P (0.2 eq.), Et3N (2 eq.), CO (1 atm), CH3OH, DMF, 40 °C, 49% (over two steps); (n) K2CO3 (2 eq.), CH3OH, rt; (o) 3 M NaOH (3 eq.), H2O2 (10 eq.), CH3OH, 0 °C→ rt, 61% (over two steps); (p) Me3SiCl (2.5 eq.), LiN(SiMe3)2 (2.1 eq.), THF, –78 °C, then NIS (1.1 eq.); (q) DMDO (0.05 M in acetone, 6 eq.), CH2Cl2, rt; (r) LiOH (2 eq.), THF, rt, 40% (over three steps). DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; p-TsOH = para-toluene sulfonic acid; DIBAL-H = diisobutylaluminum hydride; DMSO = dimethyl sulfoxide; TosMIC = para-toluenesulfonylmethyl isocyanide; DMF = dimethylformamide; NIS = N-iodosuccinimide; DMDO = dimethyldioxirane.
