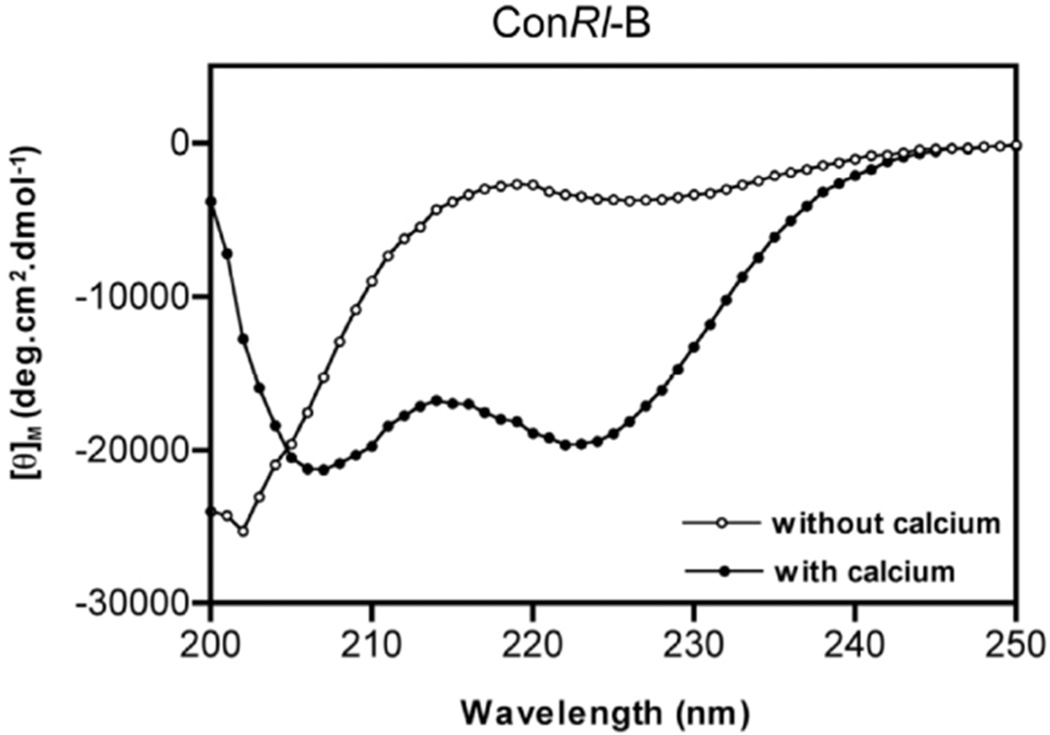
Figure 5.
Circular dichroism spectra of ConRl-B. Spectra were recorded with (or) without 2mM CaCl2 containing 10 mM HEPES buffer at pH 7.0 and shown is an average spectra obtained from five independent scans (n=5). The dual minima at 208 and 222nm, in the presence of calcium, suggest that ConRl-B adopts helical conformation. Estimated percentage of helicity of peptide in the absence of calcium is 10% and in the presence of calcium is 59%.