Abstract
Toxoplasmosis is a serous parasitic zoonosis caused by the protozoan Toxoplasma gondii worldwide. Human beings acquire the disease by eating infected meat containing T. gondii cysts, by ingesting water or vegetables contaminated with oocysts shed in the feces of an infected cat, and by transmission from mother to fetus. Cerebral toxoplasmosis is one of the most serious complications in immunocompromised individuals such as HIV-infected patients, with a high mortality rate, whereas the incidence of cerebral toxoplasmosis is extremely rare in immunocompetent persons. Due to the low incidence and the high rate of misdiagnosis, cerebral toxoplasmosis was occasionally described in sporadic cases.1 Furthermore, the diagnosis of cerebral toxoplasmosis is rather difficult because the clinical manifestations are non-specific and are not sufficiently characteristic for a definite diagnosis. It mimics several other infectious diseases or primary central nervous system (CNS) tumor.2 In the present study, we reported an exceedingly rare cerebral toxoplasmosis with obvious space-occupying lesion occurring in the left temporal lobe of an immunocompetent adult patient. To our knowledge, this is the first report of successful treatment of acquired cerebral toxoplasmosis in China.
Keywords: Cerebral toxoplasmosis, Differential diagnosis, Treatment
Case Description
A 33-year-old male patient was admitted to the hospital with a 9-month history of dysphasia and numbness of his left limb for 1 month. This patient had a history of raising pet cats for several years, but he did not have the history of pulmonary tuberculosis (TB), diabetes mellitus (DM), or HIV infection. His chest X-ray and electrocardiogram were normal. His physical examination was remarkable for a diminished superficial sensation in the right upper and lower extremities. Expressive aphasia was present with progressive deterioration. A positive Babinski sign on the right was also observed in this case. Funduscopic examination demonstrated the papilledema of optic fundi. Laboratory assays showed a peripheral leukocyte count of 9.1 × 109/l and a slightly elevated lymphocytes percentage of 42% (normal: 20–40%). Plain computer tomography (CT) scans disclosed an isodensity lesion encapsulated by surrounding edema (data not shown). Magnetic resonance imaging (MRI) revealed a space-occupying lesion with isointense in T1 and hypointensity in T2-weighted imaging and adjacent cerebral edema. Mushroom-shaped lesions with irregular-enhanced rim were observed in MRI after contrast injection (Fig. 1A and B). Serum and cerebrospinal fluid (CSF) antibodies against tissue-dwelling parasites (Paragonimus skrjabini, Schistosoma japonicum, Spirometra erinaceieuropaei, Taenia solium, and Echinococcus granulosus) were also assayed by enzyme-linked immunosorbant assay (ELISA) or immunofluorescence test (IFT),3 and were negative. Surprisingly, serum and CSF anti-Toxoplasma IgG antibodies were positive. Tumor makers such as CEA, AFP, and FRP were undetectable in CSF.
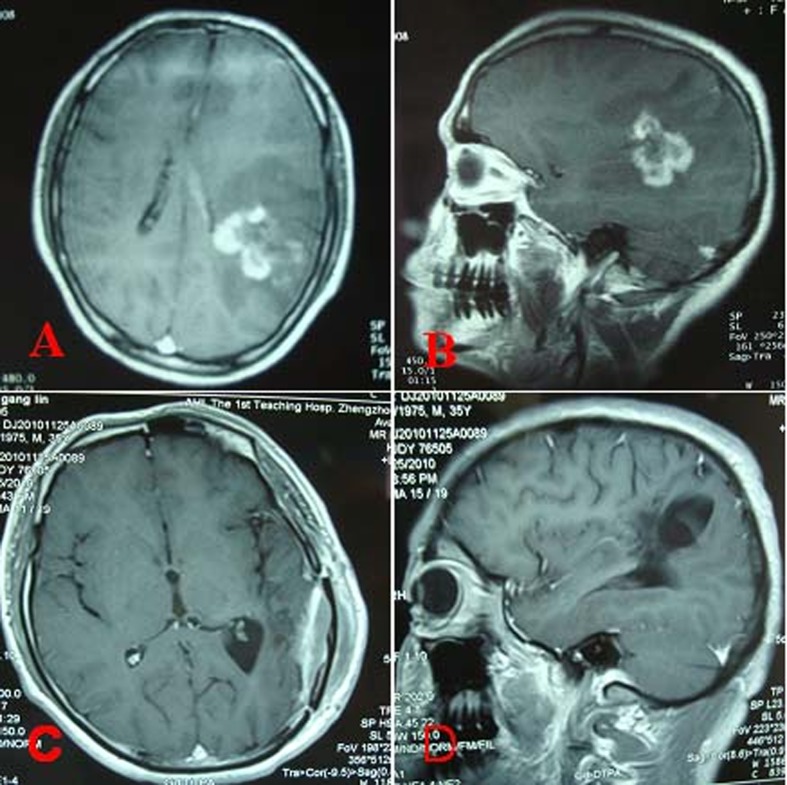
Figure 1.
Preoperative and postoperative MRI of a patient with cerebral toxoplasmosis. Axial (A) and sagittal (B) MRI with gadolinium showing a mushroom-shaped ring-enhancing lesion and perilesional brain edema in the left temporal lobe. Postoperative axial (C) and sagittal (D) MRI scan (with contrast) showing total excision of the lesion with no recurrence 2.5 years after surgery.
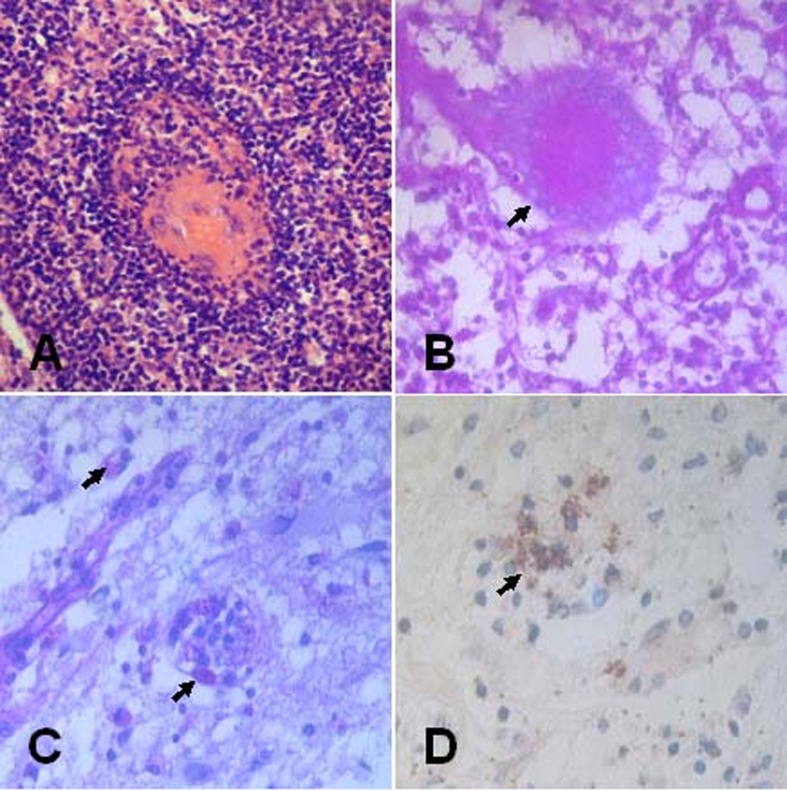
The patient underwent total pseudotumor removal and decompressive craniectomy on 7 April 2008. The patient had a smooth postoperative course and his preoperative symptoms improved gradually. Pathological specimens were routinely fixed with formaldehyde, embedded in paraffin, and sectioned for hematoxylin–eosin (HE) staining. Histological examination demonstrated the presence of protozoan parasite in a pseudocyst structure and a capsule consisted of lymphocytes, plasmocytes, and macrophages among the inflammatory infiltrate tissues (Fig. 2A). A typical ring-like pseudocyst structure was surrounded by different inflammatory cells (Fig. 2B). Toxoplasma infection was verified by periodic acid–Schiff (PAS) staining and specific streptavidin-peroxidase immunohistochemical staining of surgical specimen using Toxoplasma gondii Ab-1 (Fig. 2C and D). After initiation of intravenous azithromycin (AZM) 500 mg for 2 weeks, the patient was subsequently treated with oral AZM (250 mg twice daily) for another 4 weeks. His symptoms and radiological findings improved dramatically. Follow-up MRI showed no recurrence at 2.5 years after operation (Fig. 1C and D).
Figure 2.
Histopathological sections of surgical specimen of a patient with cerebral toxoplasmosis, showing the protozoan parasites in a pseudocyst structure surrounded by lymphocytes, plasmocytes, and macrophages. (A and B) The typical ring-like pseudocyst was surrounded by different inflammatory cells (HE, ×400). (C and D) The surgical specimen was positively reacted to PAS staining and to streptavidin-peroxidase immunohistochemical stains using Toxoplasma gondii Ab-1, respectively (arrow indicating, ×400).
Discussion
Until now, few cases of cerebral toxoplasmosis with mass effect have been reported. The transmission of this parasite to the fetus during early pregnancy can lead to the development of a congenital infection. The case with congenital toxoplasmosis mimicking a cerebral tumor was reported by Hervei and Simon.4 However, a majority of patients with cerebral toxoplasmosis are closely linked to immunosuppressive diseases, especially HIV.5–8 Toxoplasma infection in human beings is caused mainly by the ingestion of the oocyst in feces of infected cats and cysts or pseudocyst in infected meats. The patient reported in this paper has raised pet cats for several years, ingestion of the oocyst from cats is likely the source of infection. Furthermore, this patient also might acquire the Toxoplasma infection by consumption of the cyst- or pseudocyst-containing raw or undercooked meats.
Preoperative diagnosis of cerebral toxoplasmosis is considerably difficult based on the clinical symptoms and CT scan, because this disease on imaging closely mimics central nervous system (CNS) lymphomas, primary and metastatic CNS tumors, or other intracranial infectious diseases including tuberculomas and abscesses based on previous literatures (Table 1).8–10 Indeed, the rate of misdiagnosis accounted for 46.1% of parasitic encephalopathy, and only one cerebral toxoplasmosis was confirmed by the histological examination in 78 Chinese cases.11 Recently, MRI has played a crucial role in the differential diagnosis of Toxoplasma granuloma. Several reports indicated that a MRI feature on postcontrast T1-weighted sequences considered the ‘eccentric target sign’ as pathognomonic of toxoplasmosis.12–14 However, the characteristic finding of MRI does not appear in all cases. The present case was characterized by the mushroom-shaped lesion with central necrotizing abscesses. In addition, a diffusion weighted magnetic resonance (DW-MR) may be helpful for distinguishing intrinsic tumors from infective lesions. Unfortunately, due to lack of DW-MR equipment in our hospital in 2008, cerebral toxoplasmosis was not suspected before operation. The definite diagnosis of cerebral toxoplasmosis was subsequently confirmed by histological examination. The protozoan parasite was found in a pseudocyst structure, encapsulated by the inflammatory infiltrates consisting of lymphocytes, plasmocytes, and macrophages. Cerebral toxoplasmosis was further confirmed by a positive specific immunohistochemical staining of T. gondii in surgical specimen.
Table 1. Clinical features of the misdiagnosed cerebral toxoplasmosis reported in the literature.
| Case No. | Author | Year | Age/sex | Clinical features | Lesion location | Initial diagnosis | Treatment | Outcome |
| 1 | Connor et al.1 | 1984 | 15/female | Fever, headache | Right frontal lobe | Brain abscess | Chemotherapy | Excellent |
| 2 | Poon et al.9 | 1994 | 32/male | Lethargy, anorexia, midepigastric pain | Pineal region | Brain tumor | Chemotherapy | Dead |
| 3 | Nakazaki et al.8 | 2000 | 63/male | Right hemiparesis | Left frontal lobe, right temporal Lobe | Multiple brain tumor | Surgery | Deteriorated |
| 4 | Nakazaki et al.8 | 2000 | 40/male | Epilepsy, coma, left hemiparesis | Right frontoparietal Lobe | Metastatic brain tumor | Surgery | Dead |
| 5 | Nakazaki et al.8 | 2000 | 49/male | Epilepsy | Right parietal temporal lob | None-available | Chemotherapy | Excellent |
| 6 | Nakazaki et al.8 | 2000 | 64/male | Left hemiparesis | Right basal ganglia | None-available | Chemotherapy | Deteriorated |
| 7 | Ozgiray et al.5 | 2007 | 27/female | Headache, confusion, left hemiparesis | Left occipital right parietal lobe | Metastatic brain tumor | Surgery | Excellent |
| 8 | Valenta et al.10 | 2009 | 40/male | None-available | None-available | Brain tumor | Surgery | Excellent |
| 9 | Doraiswamy et al.2 | 2010 | 11/male | Lethargy, headache, fever, recurrent generalized seizures | Left basal ganglia | Brain tuberculoma | Chemotherapy | Excellent |
| 10 | Present case | 2014 | 33/male | Numbness of left limb, dysphasia | Left temporal lobe | Glioma or lymphoma | Surgery | Excellent |
Microsurgery should be performed rapidly when cerebral toxoplasmosis developed a remarkable space-occupying lesion. The clinical symptoms occasionally deteriorate immediately after admission if the patient suffered from the delay in correct diagnosis and effective treatment. The early diagnosis of cerebral toxoplasmosis is closely associated with a good prognosis. Therefore, effective anti-Toxoplasma therapy should be started as soon as cerebral toxoplasmosis is suspected. The routine drug for treatment of cerebral toxoplasmosis is trimethoprim–sulfamethoxazole. However, considering the severe renal toxicity, an alternative drug called AZM has been administrated effectively for cerebral toxoplasmosis. Consequently, we chose to initiate the intravenous delivery of AZM for 2 weeks, subsequently replaced with oral AZM (250 mg twice daily for 4 weeks). The total therapy lasted 6 weeks to avoid the recurrence of Toxoplasma infection. During the course of treatment, the patient’s symptoms and radiological findings improved dramatically. A follow-up MRI showed complete resolution of the lesion 2.5 years after operation.
In summary, the solitary cerebral toxoplasmosis mimicking the tumor is extremely rare in immunocompetent patients. An isolated cerebral toxoplasmosis should be highly suspected in a patient with the following conditions: a history of long-term contact with pet cats, typical MRI imaging of a mushroom-shaped and ring-enhancing lesion, and anti-T. gondii antibody positivity of serum and CSF samples. However, the definite diagnosis should be confirmed by histopathological examination of surgical specimen, especially by PAS staining and specific immunohistochemical staining with T. gondii Ab-1. Early surgery and anti-Toxoplasma therapy should also be emphasized for rare neurotoxoplasmosis. Early operation and definite diagnosis as well as immediate chemotherapy will provide a satisfactory prognosis for cerebral toxoplasmosis.
Disclaimer Statements
Contributors ZHL and FYG collected and analyzed the data, and drafted the manuscript. FYG did the surgery. ZHL, ZQW and JC performed the parasitological diagnosis. ZQW and JC revised the manuscript. All authors read and approved the final manuscript.
Funding The Natural Scientific Foundation of China
Conflicts of interest The authors have no conflicts of interest.
Ethics approval Not applicable.
Acknowledgments
This study was supported in part by the Natural Scientific Foundation of China (No. U1204807 and No. 81172612).
References
- 1.Connor E, Menegus M, Cecalupo A, Gigliotti F. Central nervous system toxoplasmosis mimicking a brain abscess in a compromised pediatric patient. Pediatr Infect Dis. 1984;3:552–5. doi: 10.1097/00006454-198411000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Doraiswamy V, Vaswani RK, Lahiri KR, Kondekar SS. Neurotoxoplasmosis mimicking intracranial tuberculoma. J Postgard Med. 2010;56:31–4. doi: 10.4103/0022-3859.62432. [DOI] [PubMed] [Google Scholar]
- 3.Wang ZQ, Cui J, Wang Y. Persistent febrile hepatomegaly with eosinophilia due to hepatic capillariasis in China. Ann Trop Med Parasitol. 2011;105:469–72. doi: 10.1179/1364859411Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hervei S, Simon K. Congenital toxoplasmosis mimicking a cerebral tumor. Special aspects in serodiagnostics of connatal toxoplasmosis (author’s transl). Monatsschr Kinderheilkd. 1979;127:43–7. [PubMed] [Google Scholar]
- 5.Ozgiray E, Oner K, Ovul I. HIV related toxoplasmic encephalitis mimicking multiple metastasis: case report. Turk Neurosurg. 2007;17((3)):207–10. [PubMed] [Google Scholar]
- 6.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–8. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 7.Naqi R, Azeemuddin M, Ahsan H. Cerebral toxoplasmosis in a patient with acquired immunodeficiency syndrome. J Pak Med Assoc. 2010;60:316–8. [PubMed] [Google Scholar]
- 8.Nakazaki S, Saeki N, Itoh S, Osato K, Watanabe O, Hamada N, et al. Toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome–four case reports. Neurol Med Chir (Tokyo). 2000;40:120–3. doi: 10.2176/nmc.40.120. [DOI] [PubMed] [Google Scholar]
- 9.Poon TP, Behbahani M, Matoso I, Kim B. Pineal toxoplasmosis mimicking pineal tumor in an AIDS patient. J Natl Med Assoc. 1994;86:550–2. [PMC free article] [PubMed] [Google Scholar]
- 10.Valenta Z, Förstl M, Kapla J, Kohout A. Toxoplasmic encephalitis in an HIV patient. Klin Mikrobio Infekc Lek. 2009;15:80–2. [PubMed] [Google Scholar]
- 11.Wang SM, Yang FF, Huang YX, Shi GF, Weng XH. Clinical analysis of 78 cases of parasitic encephalopathy. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:245–8. [PubMed] [Google Scholar]
- 12.Gupta A, Raja A, Mahadevan A, Shankar SK. Toxoplasma granuloma of brainstem: a rare case. Neurol India. 2008;56:189–91. [PubMed] [Google Scholar]
- 13.Kumar GG, Mahadevan A, Guruprasad AS, Kovoor JM, Satishchandra P, Nath A. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31:1469–72. doi: 10.1002/jmri.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masamed R, Meleis A, Lee EW, Hathout GM. Cerebral toxoplasmosis: case review and description of a new imaging sign. Clin Radiol. 2009;64:560–3. doi: 10.1016/j.crad.2008.09.016. [DOI] [PubMed] [Google Scholar]