Abstract
Inadequate magnesium (Mg) intake is a widespread problem, with over 50% of women of reproductive age consuming less than the Recommended Dietary Allowance (RDA). Because pregnancy increases the requirement for Mg and the beneficial effects of magnesium sulfate for preeclampsia/eclampsia and fetal neuroprotection are well described, we examined the outcomes of Mg deficiency during pregnancy. Briefly, pregnant Swiss Webster mice were fed either control or Mg-deficient diets starting on gestational day (GD) 6 through euthanasia on GD17. Mg-deficient dams had significantly reduced weight gain and higher plasma adipokines, in the absence of inflammation. Livers of Mg-deficient dams had significantly higher saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) and lower polyunsaturated fatty acids (PUFAs), including docosahexaenoic acid (DHA) (P < 0.0001) and arachidonic acid (AA) (P < 0.0001). Mechanistically, Mg deficiency was accompanied by enhanced desaturase and elongase mRNA expression in maternal livers along with higher circulating insulin and glucose concentrations (P < 0.05) and increased mRNA expression of Srebf1 and Chrebp, regulators of fatty acid synthesis (P < 0.05). Fetal pups exposed to Mg deficiency were growth-restricted and exhibited reduced survival. Mg-deficient fetal livers showed lower MUFAs and higher PUFAs, with lower desaturase and elongase mRNA expression than controls. In addition, DHA concentrations were lower in Mg-deficient fetal brains (P < 0.05). These results indicate that Mg deficiency during pregnancy influences both maternal and fetal fatty acid metabolism, fetal growth and fetal survival, and support better understanding maternal Mg status before and during pregnancy.
INTRODUCTION
There is substantial evidence that nutritional status during pregnancy significantly affects maternal health and pregnancy outcomes. Magnesium (Mg) deficiency is common and can be attributed to inadequate intake, impaired absorption, enhanced losses and/or increased biological requirements (1,2). It has been reported that >50% of women in their reproductive years do not consume the Recommended Dietary Allowance (RDA) for Mg (310 mg/day) (1,2). In addition to reduced consumption of Mg-containing foods, women are at risk for impaired Mg absorption and/or increased Mg losses because of obesity, diabetes, conditions of intestinal malabsorption, hormonal imbalances, the use of alcohol and/or drugs that compromise renal Mg handling (for example, nonsteroidal antiinflammatory agents, diuretics, antibiotics, excess calcium supplementation) and hyperemesis gravidarum, as well as excessive vomiting during pregnancy (3,4). Mg is a required nutrient; it is essential for the activity of ATP and is a cofactor for over 300 biological enzymes, including those involved in glycolysis and lipid metabolism, as well as the synthesis of protein, RNA and DNA (3,4). During pregnancy, the daily requirement for Mg increases by approximately 30% to support the rapid growth of maternal, gestational and fetal tissues. Despite this greater demand, pre-natal vitamin supplements rarely provide >25–35% of the RDA for Mg.
Suboptimal nutrient consumption during pregnancy can have negative consequences on maternal and fetal health, some of which can linger beyond pregnancy. The maternal liver has been identified as the main source of polyunsaturated fatty acids (PUFAs), specifically arachidonic acid (AA) and docosa-hexaenoic acid (DHA) (5), which are transferred across the placenta (6) and essential for the healthy development of the fetal brain and central nervous system (7,8). On the basis of the well- documented inadequate intakes of Mg before and during the reproductive years (1), the increased requirement for Mg during pregnancy, and the links between Mg deficiency and enhanced inflammation, aberrant lipid metabolism and insulin resistance (3,4,9–12), we examined the effects of Mg deficiency during pregnancy on maternal and fetal outcomes in mice.
MATERIALS AND METHODS
Mouse Model of Mg Deficiency During Pregnancy
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research (IACUC #2010-018) before commencement and complied with the Guide for the Care and Use of Laboratory Animals (13). After a 1-wk acclimatization period, outbred Swiss Webster female mice (9–14 wks old; Taconic Farms, Germantown, NY, USA) were mated with normal Swiss Webster male mice. Female mice were weighed just before mating and on gestation day (GD) 1, GD6, GD12 and GD17. On GD6, pregnant dams were randomly assigned to receive either the control AIN-76–based diet [containing 500 mg/kg elemental Mg or 100% of the recommended Mg for mice (14), n = 12] or the Mg-deficient diet [containing 50 mg/kg elemental Mg (n = 14); Harlan Teklad, Madison, WI, USA], as previously described (15). The diet was administered beginning on GD6 because, if initiated earlier (for example, GD1 or GD3), few viable fetuses were observed on GD17. Dams continued their respective diets ad libitum throughout pregnancy and were euthanized on GD17 by CO2 asphyxiation and exsanguination by cardiac puncture by using heparinized needles/syringes between 9:00 and 11:00 am (under nonfasting conditions). Fetal pups were weighed and euthanized by decapitation; fetal blood was collected in heparinized capillary tubes. The 10th percentile for fetal weight (of the control group) was determined using Microsoft Excel (percentile function). Cell-free amniotic fluid, fetal plasma, fetal liver and fetal brain samples of a single dam were pooled and snap frozen in liquid N2. All samples were stored at −80°C until use.
Assessment of Magnesium (Mg) Concentrations
Quantitative determination of ionized Mg2+ concentrations in maternal and fetal plasma, as well as amniotic fluid, was performed by using the QuantiChrom™ Magnesium Assay Kit (Bioassay Systems, Hayward, CA, USA), according to the manufacturer’s guidelines.
Evaluation of Cytokines, Adipokines, Glucose and Insulin
Cytokines
Maternal and fetal livers and plasma, as well as amniotic fluids, were analyzed for multiple cytokines by using the mouse 7-plex proinflammatory cytokine/chemokine assay kit (interleukin [IL]-1β, IL-12p70, interferon [IFN]-γ, IL-6, CXCL1 [CXC-motif ligand 1 or GRO/KC], IL-10 and tumor necrosis factor [TNF]) (Meso Scale Discovery [MSD], Rockville, MD, USA), as previously described (16). Except for IL-12p70 (lower limit 25 pg/mL) and IL-10 (lower limit 11 pg/mL), the lower limits of detection for the analytes in this assay were between 0.38 and 5.0 pg/mL. The R2 value for each standard curve was between 0.997 and 0.999. Samples being compared were run on the same plate and the percent coefficient of variation of control replicates run on the same plate was between 3% (IL-10) and 12% (CXCL1). Liver cytokine data were corrected for protein concentration (determined by Bio-Rad protein assay; Bio-Rad, Hercules, CA, USA) and expressed as pg/g.
Adipokine, insulin and glucose measurements
Maternal plasma and amniotic fluid adiponectin concentrations were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s directions. Maternal plasma and amniotic fluid insulin and leptin concentrations were determined by using MSD (as described above for cytokines). Maternal and fetal plasma glucose concentrations were measured by using the BioVision Glucose Assay Kit (Milipitas, CA, USA), according to the manufacturer’s guidelines.
Fatty Acid Analyses
Lipids were extracted from maternal plasma, maternal and fetal livers and fetal brains by using the method of Folch et al. (17). Total plasma free fatty acids (FFAs) and individual fatty acids (FAs) of the triglyceride fraction from the livers and the FA profile of the phospholipid fractions from the fetal brains were quantified. Briefly, individual lipid classes of the chloroform phase were separated by thin-layer chromatography, and FAs were isolated and methylated, as previously described (18). The methylated FAs were analyzed by using an Agilent 7890A gas chromatograph. FA methyl esters were identified by comparing the retention times to those of known standards. Tissue FAs were normalized to tissue weight and expressed as percent of total fraction (mg/100 mg).
Expression of Markers of FA Synthesis/ FA Metabolism
Markers of FA synthesis and metabolism in maternal and fetal livers were assessed by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) methods. RNA was isolated from 50-mg frozen maternal and fetal livers by using the RNeasy® Plus Universal Mini Kit with DNase treatment (Qiagen, Foster City, CA, USA). The purity/ concentration of total RNA was assayed by using the Nanodrop spectrophotometer; RNA samples had 260:280 and 260:230 ratios ≥1.9 qRT-PCRs by using specific primers (designed by the Roche Universal ProbeLibrary Assay Design Center [http://lifescience.roche.com/shop/products/universal-probelibrary-system-assay-design; Roche Diagnostics Corporation, Indianapolis, IN, USA] and synthesized by Thermo Fisher Scientific, Waltham, MA, USA) for desaturase- and elongase-related genes: D5d, D6d, Scd1, Elovl1 (variants 1–3), Elovl2, Elovl4 (variants 1–2), Elovl5, Elovl6 and other genes involved in FA synthesis: Chrebp (also known as Mlxipl), Srebf1, Fasn and Acaca were performed in triplicate by using the Eurogentec One-Step RT qPCR master-mix (Eurogentec North America, San Diego, CA, USA), 100 ng RNA, Roche Universal ProbeLibrary and the Roche LightCycler 480®, as previously described (19). See Supplementary Table S1 for primers and probes. Relative changes in mRNA expression were calculated as fold changes (versus mouse Hprt1 as the housekeeping gene) by using the comparative Ct (ΔΔCt) method (20).
Indices of FA desaturation (monounsaturated fatty acid [MUFA]/saturated fatty acid [SFA] ratio) and elongation were determined by using product-to-substrate ratios, as previously described (21). Hepatic sterol regulatory element binding protein-1 (SREBP-1) and carbohydrate-responsive element binding protein (ChREBP) concentrations were determined by Western blotting by using specific antibodies: anti–SREBP-1 (H-160; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-ChREBP (NB400-135; Novus Biologicals, Littleton, CO, USA).
Statistical Analyses
All data are presented as means ± standard deviation (SD), unless otherwise indicated. qRT-PCR results (specific mRNA/Hprt1 [housekeeping gene] mRNA ratios) are expressed as mean fold change (± SD) relative to the control group. Except for fetal death data, all data were analyzed by using either unpaired t tests (when there were equal variances) or unpaired t tests with the Welch correction (when there were unequal variances) by using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) to compare controls versus Mg-deficient animals. Fetal death data was analyzed by using the Exact Mann-Whitney test to compare the percent of nonviable fetal pups per litter among the control group versus the Mg-deficient group by using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Effect of Maternal Mg Deficiency on Maternal Weight Gain, Fetal Growth and Fetal Survival
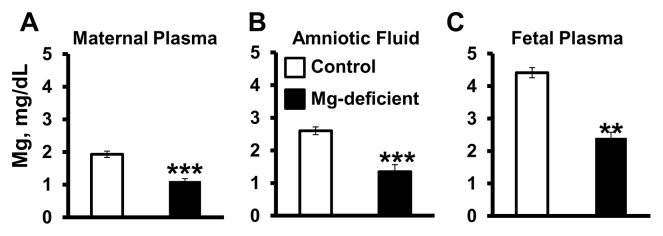
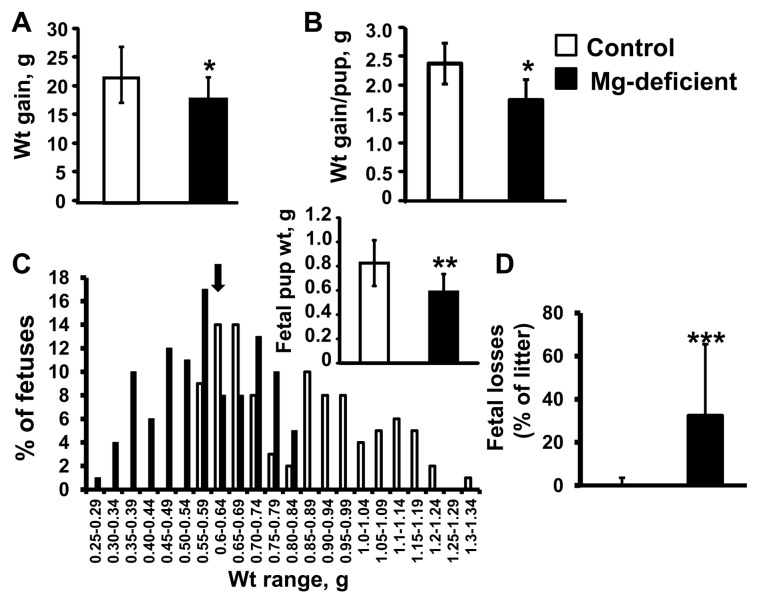
Consumption of the Mg-deficient diet by the mouse dams beginning on GD6 and continuing through GD17 (11 d) significantly reduced Mg concentrations in the maternal plasma, amniotic fluid and fetal plasma by approximately 40–45% (Figure 1, P < 0.01). A concentration gradient for Mg from the maternal side (low) to the fetal side (high) was observed (Figure 1). Dams fed the Mg-deficient diet gained significantly less weight throughout pregnancy compared with the control-fed dams (P < 0.05) (Figure 2A). In addition, maternal weight gain per fetal pup (that is, corrected for litter size) was reduced by approximately 20% in the Mg-deficient group (P < 0.05) (Figure 2B). Litter sizes were not significantly different between the two groups. Fetal pups exposed to Mg deficiency in utero weighed approximately 30% less than pups exposed to the control diet on E17 (P < 0.01) (Figure 2C), with approximately 60% of Mg-deficient pups weighing below the 10th percentile of the control pup weight (Figure 2C). Fetal losses (% nonviable pups per litter on GD17) were significantly greater among the Mg-deficient dams versus controls (P < 0.001) (Figure 2D).
Figure 1.
Mg deficiency reduces Mg concentrations in maternal and fetal plasma and amniotic fluid. Mg concentrations (mg/dL) in maternal plasma (A), amniotic fluid (B) and fetal plasma (C) were determined on GD17 after consumption of either control or Mg-deficient diets. Values are means ± SD; n = 12 (control) and n = 14 (Mg deficient) per treatment. **P < 0.01 versus control, ***P < 0.001 versus control.
Figure 2.
Mg deficiency reduces maternal and fetal weight gain and fetal survival. Maternal weight (wt) gain between GD1 and GD17 (A), maternal weight gain corrected for litter size (weight gain per pup) on GD17 (B) and the distribution of control and Mg-deficient E17 mouse fetal weights (expressed as a percentage of fetal pups for each weight category) are shown. The arrow indicates the 10 percentile cutoff for control fetal weight; inset shows overall mean fetal pup weight per group (C). Fetal losses (expressed as percentage of litter) determined on GD17 (D). Values are means ± SD; n = 12 (control) and n = 14 (Mg deficient) per group. *P < 0.05 versus control, **P < 0.01 versus control, ***P < 0.001 versus control.
Mg Deficiency Is Not Associated with Maternal or Fetal Inflammation
Consumption of an Mg-deficient diet did not affect maternal plasma cytokines (IL-1β, IL-6, CXCL1, IL-12p70, IFNγ or IL-10) or liver cytokines (IL-1β, IL-6, CXCL1, IL-12p70 or IFNγ [IL-10 and TNF were undetectable]) when assessed on GD17 (Supplementary Table S2). Likewise, amniotic fluid, fetal plasma and fetal livers obtained from the fetal pups exposed to Mg deficiency showed no significant differences in cytokine concentrations compared with controls (Supplementary Table S2). With the exception of CXCL1, our data were very low values with considerable variation.
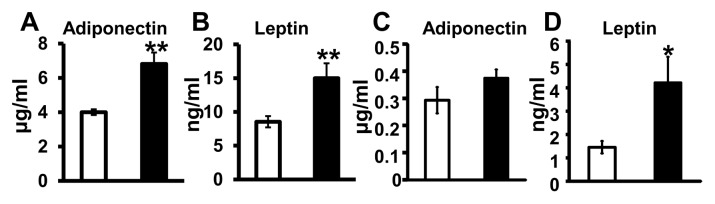
Mg Deficiency during Pregnancy Affects Circulating Adipokine Concentrations
Maternal plasma adiponectin and leptin concentrations were significantly higher in the Mg-deficient dams when compared with control dams (P < 0.01) (Figures 3A, B). Mg status did not significantly affect adiponectin concentrations in the amniotic fluid (Figure 3C). However, leptin concentrations in the amniotic fluid were significantly higher in the Mg-deficient mice when compared with controls (P < 0.05) (Figure 3D).
Figure 3.
Mg deficiency during pregnancy increases maternal circulating adipokines. Maternal plasma adiponectin (A) and leptin (B) concentrations and amniotic fluid adiponectin (C) and leptin (D) concentrations determined on GD17. Values are means ± SEM (standard error of the mean); n = 10 (control, □) and n = 11 (Mg deficient, ■) mice per group. *P < 0.05 versus control, **P < 0.01 versus control.
Mg Deficiency during Pregnancy in Mice Was Accompanied by Higher Maternal Plasma FFAs and Significant Changes in Maternal and Fetal Hepatic FA Profiles
Following Mg deficiency during pregnancy, dams had higher total plasma FFAs when compared with control dams (Mg deficient: 102.6 ± 32.6 μg/mL versus controls: 74.0 ± 11 μg/mL, P < 0.01). Although total maternal liver triglyceride concentrations were similar among Mg-deficient and control dams (Mg deficient: 5.8 ± 3.1 μg/mg versus controls: 4.6 ± 1.9 μg/mg), the overall maternal liver triglyceride-FA profile was significantly altered after Mg deficiency (Table 1). Mg deficiency was accompanied by significantly greater SFAs (P < 0.01) and MUFAs (P < 0.05) and significantly lower PUFAs (P < 0.01), as well as PUFA/SFA (P < 0.05) and n-3:n-6 FA ratios (P < 0.05, Table 1). More specifically, Mg- deficient dams had higher percentages of 14:0 (by >100%), 16:1 (by 79%) and 18:1n-9 (by 15%) liver FAs and significantly lower percentages of 18:2n-6 (by 20%), 18:3n-3 (by 31%), 18:3n-6 (by 50%), 20:4n-6 (AA, by 56%) and 22:6n-3 (DHA, by 87%) liver FAs (Table 1).
Table 1.
Mg deficiency during pregnancy alters maternal and fetal hepatic fatty acid profiles.
| Maternal | Fetal | |||
|---|---|---|---|---|
|
|
|
|||
| Control | Mg deficient | Control | Mg deficient | |
| FA (mg per 100 mg liver) | ||||
|
| ||||
| 14:0 | 0.36 ± 0.22 | 0.74 ± 0.13** | 1.75 ± 0.21 | 1.55 ± 0.19 |
| 16:0 | 30.2 ± 0.86 | 30.8 ± 2.55 | 27.8 ± 1.00 | 26.3 ± 0.58* |
| 16:1 | 2.02 ± 0.34 | 3.63 ± 1.02** | 5.21 ± 0.30 | 4.74 ± 0.68 |
| 18:0 | 4.46 ± 0.48 | 4.8 ± 0.82 | 7.89 ± 0.87 | 8.75 ± 0.94 |
| 18:1n-7 | 2.70 ± 0.25 | 3.01 ± 0.59 | 3.90 ± 0.23 | 3.37 ± 0.38* |
| 18:1n-9 | 30.3 ± 2.54 | 34.7 ± 2.68** | 38.1 ± 1.60 | 32.8 ± 2.48** |
| 18:2n-6 | 24.2 ± 2.74 | 19.3 ± 4.96* | 13.8 ± 1.90 | 19.4 ± 2.62*** |
| 18:3n-6 | 0.97 ± 0.18 | 0.48 ± 0.58* | 0.36 ± 0.36 | 0.78 ± 0.35* |
| 18:3n-3 | 0.54 ± 0.37 | 0.37 ± 0.99* | 0.23 ± 0.34 | 0.87 ± 0.46* |
| 20:4n-6 | 2.80 ± 0.57 | 1.24 ± 0.44*** | 0.26 ± 0.27 | 0.71 ± 0.33* |
| 22:6n-3 | 1.41 ± 0.42 | 0.19 ± 0.33*** | 0.67 ± 0.05 | 0.93 ± 0.17* |
|
| ||||
| Percentage of total | ||||
|
| ||||
| MUFA | 37.0 ± 1.20 | 39.8 ± 3.06* | 47.0 ± 1.80 | 40.1 ± 3.56** |
| SFA | 33.0 ± 2.70 | 40.0 ± 3.20** | 37.4 ± 1.80 | 37.1 ± 1.73 |
| PUFA | 30.0 ± 2.96 | 20.2 ± 6.07** | 15.6 ± 2.67 | 22.8 ± 4.17** |
|
| ||||
| Ratio | ||||
|
| ||||
| PUFA:SFA | 0.86 ± 0.10 | 0.63 ± 0.23* | 0.33 ± 0.16 | 0.61 ± 0.24** |
| n-3:n-6 FA | 0.07 ± 0.01 | 0.06 ± 0.01* | 0.06 ± 0.02 | 0.09 ± 0.01* |
|
| ||||
| Activity | ||||
|
| ||||
| Desaturation indexa | 1.00 ± 0.08 | 1.14 ± 0.09* | 1.26 ± 0.07 | 1.12 ± 0.1* |
| ELOVL6b | 1.15 ± 0.09 | 1.29 ± 0.06* | 1.65 ± 0.04 | 1.58 ± 0.09 |
Values are means ± SD; n = 8–9 per group.
Total desaturation index = (16:1n-7 + 18:1n-7 + 18:1n-9)/(14:0 + 16:0 + 18:0).
ELOVL6 (elongase 6) activity index = (18:0 + 18:1n-9)/16:0.
P < 0.05,
P < 0.01,
P < 0.0001 versus control.
Mg-deficient fetal livers had significantly lower 18:1n-9 (by 14%) and 18:1n-7 (by 13%) and higher 18:2n-6 (by 40%), 18:3n-6 (by 116%), 18:3n-3 (by 278%), 20:4n-6 (AA, by 173%) and 22:6n-3 (DHA, by 32%) (Table 1). Overall, Mg-deficient fetal livers had significantly lower hepatic MUFA and higher PUFA concentrations, a higher PUFA:SFA ratio and a higher n-3:n-6 FA ratio compared with control fetal livers (Table 1).
Fetal Brain DHA Is Lower in Mg-Deficient Fetal Pups
The only phospholipid-FA in the E17 fetal brains differentially expressed was DHA, which was significantly lower in those brains exposed to Mg deficiency in utero (controls: 11.9 ± 0.29 versus Mg deficient: 11.1 ± 0.22 mg/100 mg brain tissue; P < 0.05).
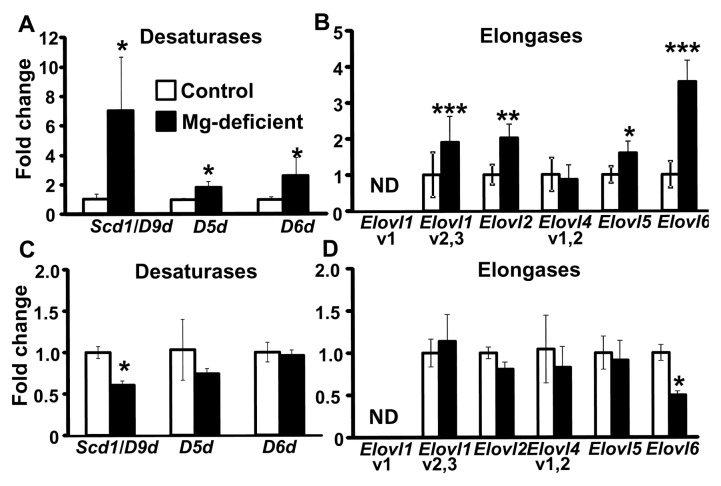
FA Desaturation and Elongation-Related Gene Expression in Maternal and Fetal Livers
Livers from the Mg-deficient dams showed a seven-fold higher expression of Scd1 (also known as D9d) mRNA and approximately two-fold higher expression of D5d and D6d mRNA compared with controls (P < 0.05) (Figure 4A). The total desaturation index for stearoyl-CoA desaturase-1 (SCD-1) activity was higher in Mg-deficient maternal livers compared with control maternal livers (P < 0.05) (Table 1). Elovl1 (variants 2 and 3), Elovl2, Elovl5 and Elovl6 mRNA expression were significantly elevated in the Mg-deficient maternal livers versus control maternal livers (P < 0.05) (Figure 4B). The ELOVL6 elongation index for 16:0 was also greater in the Mg-deficient maternal livers compared with control livers (P < 0.05) (Table 1). By contrast, Scd1 (Figure 4C) and Elovl6 mRNA expression (Figure 4D) was lower in the Mg-deficient fetal livers versus control fetal livers. The total de-saturation index for SCD-1 activity for the fetal livers exposed to Mg deficiency was significantly lower when compared with control fetal livers, but there was no difference in the elongation index (Table 1).
Figure 4.
Mg deficiency alters mRNA expression of desaturases and elongases in maternal and fetal livers. Scd1, D5d and D6d (desaturases) mRNA expression (A, C) and Elovl1 (v1 and v2,3), Elovl2, Elovl4 (v1,2), Elovl5 and Elovl6 (elongases) mRNA expression (B, D) in maternal livers (A, B) and fetal livers (C, D), respectively, are shown. Values are means (fold change) ± SD; n = 5–7 mice per group. *P < 0.05, **P < 0.01, ***P < 0.0001 versus control. ND, not detectable; v1, variant 1; v1,2, variants 1 and 2; v2,3, variants 2 and 3.
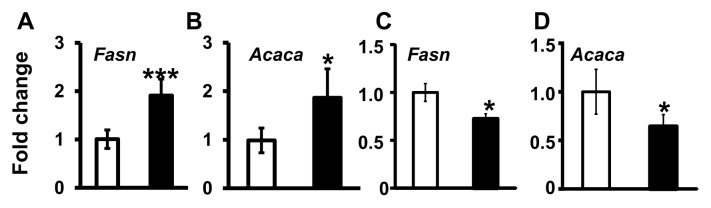
Maternal liver mRNA expression of Fasn and Acaca, regulators of SFA synthesis, were higher after Mg deficiency when compared with the controls (P < 0.05) (Figures 5A, B). By contrast, Fasn and Acaca mRNA expression were lower in the fetal livers after Mg deficiency versus control fetal livers (P < 0.05) (Figures 5C, D).
Figure 5.
Mg deficiency alters Fasn and Acaca mRNA expression in maternal livers. Fasn (A, C) and Acaca (B, D) mRNA expression in maternal mouse livers (A, B) and fetal livers (C, D), respectively, is shown. Values are means (fold change) ± SD; n = 5–7 per treatment (control, □; Mg deficient, ■). *P < 0.05, ***P < 0.0001 versus control.
Changes in Hepatic Srebf1 and Chrebp mRNA Expression
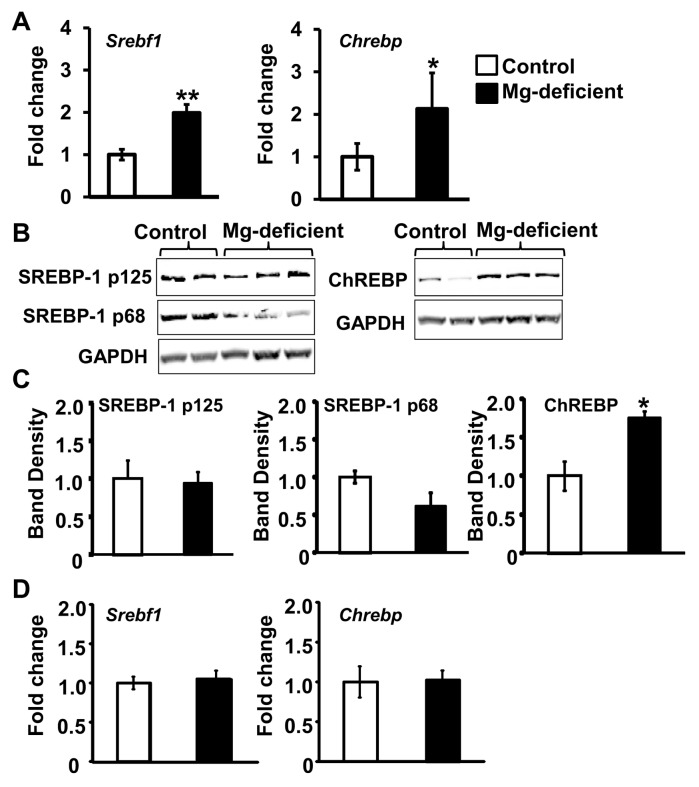
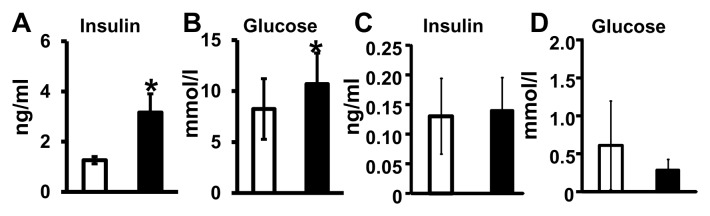
The major regulators of FA synthesis, Srebf1 and Chrebp mRNA, were higher among the Mg-deficient dams than controls (Figure 6A). Although no significant differences in SREBP-1 protein concentrations (either precursor [p125] or mature [p68] isoforms) were observed (Figures 6B, C), Mg-deficient maternal livers had significantly greater amounts of ChREBP protein compared with control livers (see Figures 6B, C). In the fetal livers exposed to Mg deficiency, no significant changes in Srebf1 or Chrebp mRNA expression were observed versus controls (Figure 6D). Elevated concentrations of the inducers of Srebf1 and Chrebp mRNA, insulin (which induces Srebf1) and glucose (which induces both Srebf1 and Chrebp mRNA) were found in the circulation of Mg-deficient dams (Figures 7A, B). On the fetal side, there were no differences in insulin or glucose concentrations after Mg deficiency (Figures 7C, D).
Figure 6.
Mg deficiency during pregnancy affects the expression of regulators of fatty acid synthesis. Srebf1 (A) and Chrebp (B) mRNA expression in maternal livers is shown. Representative Western blots are shown for maternal liver SREBP-1 proteins (precursor, 125-kDa [p125] and mature, 68-kDa [p68] forms) (B) and ChREBP protein (C), with quantitation of band densities of SREBP-1 p125, SREBP-1 p68 and ChREBP corrected for GAPDH below. Srebf1 (D) and Chrebp (E) mRNA expression in fetal livers. Values are means ± SD; n = 5–7 samples per treatment. *P < 0.05, **P < 0.01 versus control.
Figure 7.
Changes in maternal plasma glucose and insulin levels after Mg deficiency. Maternal plasma insulin (A) and glucose (B) concentrations and amniotic fluid insulin (C) and fetal plasma glucose (D) concentrations were determined. Values are means ± SD; n = 10 samples per treatment (control, □; Mg deficient, ■). *P < 0.05 versus control.
DISCUSSION
On the basis of the reduction in mouse maternal plasma Mg concentrations with respect to previously published guidelines (22,23), this model appears to produce moderate Mg deficiency. Maternal plasma Mg concentrations decline over the course of pregnancy (24). This result is consistent with the competition for Mg between the mother and fetus favoring the fetus, with higher Mg concentrations in cord blood than maternal blood (25). The competition for existing Mg between the fetuses and dams was evident by the concentration gradient from the maternal (low) to the fetal (high) side (Figure 1). Although plasma Mg concentrations may not reflect intracellular Mg availability, determination of serum/ plasma Mg concentrations is the most commonly used method to assess Mg status and adequately reflects acute Mg status resulting from altered dietary intakes (26).
Previous studies report enhanced inflammation during Mg deficiency in the absence of pregnancy in rodents and humans (11,27). We observed no increase in inflammatory cytokines or chemokines in the maternal or fetal circulation, amniotic fluid, maternal livers or fetal livers (Supplementary Table S2). However, we did not assess C-reactive protein or amyloid P, two important inflammatory mediators. The lack of enhanced inflammation in our mice may be due to elevated β-estradiol concentrations accompanying pregnancy, which have been shown to protect against Mg deficiency–induced inflammation in the nonpregnant state (28). Alternatively, Mg deficiency was not severe enough (due to length of time the dams were on the Mg-deficient diet [11 d]) to produce significant inflammatory changes.
To our knowledge, no studies have examined the effect of Mg deficiency on maternal circulating adipokines during pregnancy in mice. Leptin is upregulated during pregnancy (29), but its role in pregnancy is not well understood. Leptin is typically associated with reduced food intake, but not in pregnancy (30). A recent study revealed that higher circulating maternal leptin, an early marker of metabolic dysfunction, correlates with recurrent pregnancy losses in humans (31). In this study, higher leptin in the Mg- deficient group was accompanied by more fetal losses. Also consistent with our findings, a recent report showed that the administration of adiponectin to pregnant mice significantly restricted fetal growth (32), which was observed herein. Adiponectin improves insulin sensitivity by promoting glucose uptake and reducing hepatic gluconeogenesis (33). Thus, higher adiponectin concentrations found in Mg-deficient dams may be a compensatory mechanism to manage elevated maternal glucose and insulin concentrations. Unfortunately, because of the small amounts of fetal blood obtained, we were unable to assess fetal plasma adiponectin or leptin concentrations. Maternal nutrient deficiencies (for example, global food restriction, energy/ calorie, protein restriction and iron deficiency) can lead to fetal growth restriction or intrauterine growth restriction (IUGR). IUGR, a major cause of fetal/ neonatal morbidity and mortality, occurs when the fetal growth rate is significantly below its potential for a given gestational age. By strict definition, IUGR results when the estimated weight of the fetus is below the 10th percentile for its gestational age (34). The precise causes of IUGR are not well understood. While IUGR during maternal Mg deficiency has not been previously reported, earlier studies support the role of Mg status in fetal growth. Takaya et al. (35) reported that, in humans, Mg concentrations found in umbilical cord blood-derived platelets were significantly lower in neonates born small for gestational age (previously IUGR in utero) than the “appropriate for gestational age” control group (35). While the cause of reduced fetal growth in the Mg-deficient dams is not known, it is possible that it may result, in part, from maternal metabolic dysfunction and the need for Mg for so many important biological processes. Mg is a cofactor for enzymes involved in DNA, RNA and protein synthesis and numerous metabolic pathways required for normal growth and development. Ongoing studies in our laboratory are investigating potential mechanisms underlying fetal growth restriction in mice after exposure to Mg deficiency in utero.
Inadequate Mg intakes have been associated with metabolic syndrome in humans (4,12). Although insulin resistance was not measured in this study, Mg deficiency was associated with insulin resistance in humans and laboratory animals during the nonpregnant state (36,37). Precisely how Mg status regulates insulin sensitivity and glucose levels/tolerance is not completely understood. Mg may regulate intracellular signaling associated with glucose uptake and/or insulin signaling by serving as a cofactor for pathway-specific enzymes (38).
This study is the first to link Mg deficiency during pregnancy to dysfunctional maternal lipid metabolism and aberrant fatty acid profiles. Srebf1 and Chrebp transcribe the major overall transcriptional regulators of hepatic FA synthesis, SREBP-1 and ChREBP (39,40), respectively, which have been linked to the metabolic syndrome (41,42), as well as elevated hepatic de novo lipogenesis and fatty liver (40,43,44). The two major inducers of Srebf1 mRNA expression are insulin and glucose, whereas Chrebp mRNA expression is induced by glucose independent of insulin (40). Mg deficiency in pregnant mice was characterized by higher maternal plasma insulin and glucose concentrations as well as greater maternal hepatic Srebf1 and Chrebp mRNA expression, compared with controls (Figure 6). By contrast, DHA and other PUFAs, the negative regulators of Srebf1 (45,46) and Chrebp (47) mRNA expression, were significantly lower in Mg-deficient dams versus controls. SREBP-1 controls the expression of Scd1, D6d and Elovl5 (43), as well as Fasn and Acaca (43), which were elevated on the maternal side during Mg deficiency. ChREBP regulates both glycolytic and lipogenic genes and plays a critical role in FA synthesis (48). The protein product of Scd1, SCD-1, is the rate-limiting enzyme for MUFA synthesis (49), and its expression is primarily regulated at the level of mRNA transcription (50). Mg-deficient maternal livers showed higher ChREBP protein, but not higher SREBP-1 protein expression (Figures 6C, D). This result could be due to the instability of the mature SREBP-1 protein, which is immediately degraded (40) and/or due to our assessment of whole organ lysates rather than the nuclear fractions (where it exerts its effects) (40). Finally, providing the Mg-deficient diet for only 11 d was possibly too brief for the dams to develop fatty livers.
IUGR has been associated with aberrant FA metabolism in rat fetuses and offspring (51). In addition, previous studies have revealed the correlations between low maternal n-3 and n-6 FA plasma concentrations and poor fetal growth (52) and between maternal dietary n-3:n-6 FA ratios and childhood brain development (53). The fetus relies on the transplacental transport of maternally derived DHA (6,54), which forms a maternal-to-fetal gradient favoring the fetus (55). DHA delivery to the fetus could be compromised by the 86% reduction in maternal liver DHA after Mg deficiency. Enhanced fetal liver DHA after Mg deficiency may reflect a compensatory response to produce DHA for the fetal brain (that is, brain-sparing) and fetal growth (Figure 8). However, because the fetal liver is not capable of making sufficient DHA (6,56) and because DHA is critical for cells of the central nervous system for forming membrane phospholipids required for neurogenesis and synaptogenesis (8,57–59), a maternal deficit could have significant consequences for the fetus and offspring. In addition, maternal DHA supplementation has been associated with improved fetal growth (60).
Figure 8.
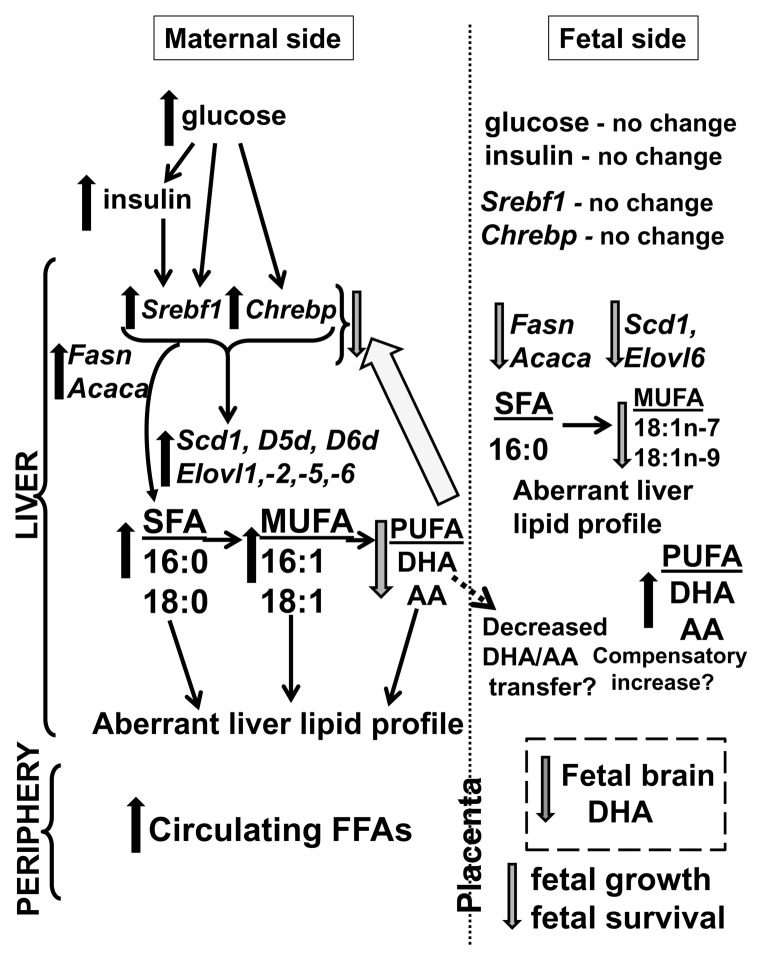
Proposed model describing the effects of maternal Mg deficiency in mice during pregnancy. On the maternal side, Mg deficiency is accompanied by higher maternal circulating glucose and insulin, positive regulators of Srebf1 and Chrebp mRNA expression, which promote Fasn and Acaca mRNA expression. Together, these promote higher hepatic SFAs, along with Scd1, D5d, D6d (desaturase) and Elovl1, Elovl2, Elovl5 and Elovl6 (elongase) mRNA expression, which lead to higher circulating FFAs and hepatic SFAs and MUFAs and lower hepatic PUFAs, specifically DHA and AA (which are negative regulators of Srebf1 and Chrebp mRNA expression). On the fetal side, Mg deficiency is accompanied by no changes in glucose or insulin concentrations, consistent with no changes in Srebf1 and Chrebp mRNA expression in the fetal liver. Inadequate supplies of maternal DHA and AA, which are critical for fetal growth and brain development, lead to lower fetal brain DHA concentrations along with a compensatory increase in fetal hepatic PUFAs (DHA and AA) and decrease in MUFAs (via decreased Fasn, Acaca, Scd1 and Elovl6 mRNA expression). Maternal and fetal metabolic dysfunction during Mg deficiency is accompanied by fetal growth restriction and increased fetal mortality.
CONCLUSION
Our data add to the results of previous work, establishing the role of Mg in maintaining metabolic function in the nonpregnant state and reveal that, in a mouse model of pregnancy, moderate Mg deficiency leads to aberrant maternal and fetal hepatic FA profiles and fetal brain DHA levels, along with changes in the expression of key transcriptional regulators involved in fatty acid metabolism (see Figure 8). Poor maternal health, including aberrant n-3 and n-6 FA profiles, can affect offspring size and health (52). Our findings support investigating whether these metabolic abnormalities foreshadow future maternal and neonatal/offspring health risks and whether this pattern can be reversed by Mg supplementation. Likewise, these findings warrant further investigation of the relationships between inadequate Mg consumption and/or Mg deficiency in humans during pregnancy and dys-regulated maternal metabolism and poor pregnancy outcomes.
Supplemental Data
ACKNOWLEDGMENTS
We acknowledge Carla Harris at the Lipid Core Laboratory of Vanderbilt University’s Mouse Metabolic Phenotyping Center (grant DK59637) who extracted and quantified the liver and brain lipids. We thank Nina Kohn, MBA, MA, of the Biostatistics Unit of the Feinstein Institute for assistance with statistical analyses. This work was supported by The Feinstein Institute and the Oxenhorn Family Foundation.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr Rev. 2012;70:153–64. doi: 10.1111/j.1753-4887.2011.00465.x. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–82. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 3.Nadler JL, Rude RK. Disorders of magnesium metabolism. Endocrinol Metab Clin North Am. 1995;24:623–41. [PubMed] [Google Scholar]
- 4.Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev. 2003;24:47–66. [PMC free article] [PubMed] [Google Scholar]
- 5.Burdge GC, Hunt AN, Postle AD. Mechanisms of hepatic phosphatidylcholine synthesis in adult rat: effects of pregnancy. Biochem J. 1994;303:941–7. doi: 10.1042/bj3030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larque E, et al. Placental transfer of fatty acids and fetal implications. Am J Clin Nutr. 2011;94:1908S–13S. doi: 10.3945/ajcn.110.001230. [DOI] [PubMed] [Google Scholar]
- 7.Coti BP, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–5. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 8.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143:S1–8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 9.Fung TT, et al. The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll Nutr. 2003;22:533–8. doi: 10.1080/07315724.2003.10719332. [DOI] [PubMed] [Google Scholar]
- 10.Nadler JL, et al. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21:1024–9. doi: 10.1161/01.hyp.21.6.1024. [DOI] [PubMed] [Google Scholar]
- 11.Weglicki WB. Hypomagnesemia and inflammation: clinical and basic aspects. Annu Rev Nutr. 2012;32:55–71. doi: 10.1146/annurev-nutr-071811-150656. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Li C, McGuire LC, Mokdad AH, Liu S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity (Silver Spring) 2007;15:1139–46. doi: 10.1038/oby.2007.628. [DOI] [PubMed] [Google Scholar]
- 13.Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 14.Subcommittee on Laboratory Nutrition; National Research Council, editor. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press; 1995. Nutrient requirements of the mouse; p. 89. [Google Scholar]
- 15.Rude RK, et al. Bone loss induced by dietary magnesium reduction to 10% of the nutrient requirement in rats is associated with increased release of substance P and tumor necrosis factor-alpha. J Nutr. 2004;134:79–85. doi: 10.1093/jn/134.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Roman A, et al. Maternal magnesium supplementation reduces intrauterine growth restriction and suppresses inflammation in a rat model. Am J Obstet Gynecol. 2013;208:383–7. doi: 10.1016/j.ajog.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5:600–8. [PubMed] [Google Scholar]
- 19.Dowling O, et al. Magnesium sulfate reduces bacterial LPS-induced inflammation at the maternal-fetal interface. Placenta. 2012;33:392–8. doi: 10.1016/j.placenta.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Cikos S, Bukovska A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;8:113. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green CD, Ozguden-Akkoc CG, Wang Y, Jump DB, Olson LK. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J Lipid Res. 2010;51:1871–7. doi: 10.1194/jlr.M004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alves SC, et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2013;28:910–6. doi: 10.1093/ndt/gfs268. [DOI] [PubMed] [Google Scholar]
- 23.Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002;28:667–79. doi: 10.1007/s00134-002-1281-y. [DOI] [PubMed] [Google Scholar]
- 24.Standley CA, Whitty JE, Mason BA, Cotton DB. Serum ionized magnesium levels in normal and preeclamptic gestation. Obstet Gynecol. 1997;89:24–7. doi: 10.1016/s0029-7844(96)00380-8. [DOI] [PubMed] [Google Scholar]
- 25.Lukacsi L, Lintner F, Zsolnai B, Somogyi J. Magnesium transport in human pregnancy (magnesium content of human gestation tissues and tissue fluids) Acta Chir Hung. 1991;32:263–8. [PubMed] [Google Scholar]
- 26.Arnaud MJ. Update on the assessment of magnesium status. Br. J. Nutr. 2008;99(Suppl 3):S24–36. doi: 10.1017/S000711450800682X. [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85:1068–74. doi: 10.1093/ajcn/85.4.1068. [DOI] [PubMed] [Google Scholar]
- 28.Nishio A, Miyazaki A, Ishiguro S, Miyao N. Sex difference of pinnal hyperemia in magnesium-deficient rats: effects of castration and administration of sex hormone. Jpn J Pharmacol. 1986;41:15–22. doi: 10.1254/jjp.41.15. [DOI] [PubMed] [Google Scholar]
- 29.Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr. 2000;130:514–21. doi: 10.1093/jn/130.3.514. [DOI] [PubMed] [Google Scholar]
- 30.Seeber RM, Smith JT, Waddell BJ. Plasma leptin-binding activity and hypothalamic leptin receptor expression during pregnancy and lactation in the rat. Biol Reprod. 2002;66:1762–7. doi: 10.1095/biolreprod66.6.1762. [DOI] [PubMed] [Google Scholar]
- 31.Baban RS, Ali NM, Al-Moayed HA. Serum leptin and insulin hormone level in recurrent pregnancy loss. Oman Med J. 2010;25:203–7. doi: 10.5001/omj.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosario FJ, et al. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590:1495–509. doi: 10.1113/jphysiol.2011.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 34.Peleg D, Kennedy CM, Hunter SK. Intrauterine growth restriction: identification and management. Am Fam Physician. 1998;58:453–7. [PubMed] [Google Scholar]
- 35.Takaya J, Yamato F, Higashino H, Kobayashi Y. Relationship of intracellular magnesium of cord blood platelets to birth weight. Metabolism. 2004;53:1544–7. doi: 10.1016/j.metabol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Huerta MG, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28:1175–81. doi: 10.2337/diacare.28.5.1175. [DOI] [PubMed] [Google Scholar]
- 37.Stefikova K, Spustova V, Sebekova K, Dzurik R. Magnesium deficiency impairs rat soleus muscle glucose utilization and insulin sensitivity. Mater Med Pol. 1992;24:215–6. [PubMed] [Google Scholar]
- 38.Takaya J, Higashino H, Kobayashi Y. Intra-cellular magnesium and insulin resistance. Magnes Res. 2004;17:126–36. [PubMed] [Google Scholar]
- 39.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, So JS, Park JG, Lee AH. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013;33:301–11. doi: 10.1055/s-0033-1358523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65–73. doi: 10.1016/j.tem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Iizuka K, Horikawa Y. ChREBP: A glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J. 2008;55:617–24. doi: 10.1507/endocrj.k07e-110. [DOI] [PubMed] [Google Scholar]
- 43.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–32. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 45.Sekiya M, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–39. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Yahagi N, et al. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J Biol Chem. 1999;274:35840–4. doi: 10.1074/jbc.274.50.35840. [DOI] [PubMed] [Google Scholar]
- 47.Dentin R, et al. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–54. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Postic C, Dentin R, Denechaud PD, Girard J. ChREBP, a transcriptional regulator of glucose and lipid metabolism. Annu Rev Nutr. 2007;27:179–92. doi: 10.1146/annurev.nutr.27.061406.093618. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv Nutr. 2011;2:15–22. doi: 10.3945/an.110.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 51.Yamada M, et al. Early onset of fatty liver in growth-restricted rat fetuses and newborns. Congenit Anom (Kyoto) 2011;51:167–73. doi: 10.1111/j.1741-4520.2011.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr. 2008;87:887–95. doi: 10.1093/ajcn/87.4.887. [DOI] [PubMed] [Google Scholar]
- 53.Bernard JY, et al. The dietary n6:n3 fatty acid ratio during pregnancy is inversely associated with child neurodevelopment in the EDEN mother-child cohort. J Nutr. 2013;143:1481–8. doi: 10.3945/jn.113.178640. [DOI] [PubMed] [Google Scholar]
- 54.Hanebutt FL, Demmelmair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr. 2008;27:685–93. doi: 10.1016/j.clnu.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Green P, Yavin E. Mechanisms of docosahexaenoic acid accretion in the fetal brain. J Neurosci Res. 1998;52:129–36. doi: 10.1002/(SICI)1097-4547(19980415)52:2<129::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Chambaz J, et al. Essential fatty acids inter-conversion in the human fetal liver. Biol Neonate. 1985;47:136–40. doi: 10.1159/000242104. [DOI] [PubMed] [Google Scholar]
- 57.Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20:2953–63. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- 58.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–38. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002;26:210–8. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 60.Carlson SE, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–15. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.