Abstract
Although many studies have examined the precedence effect (PE), few have tested whether it shows a buildup and breakdown in nonhuman animals comparable to that seen in humans. These processes are thought to reflect the ability of the auditory system to adjust to a listener's acoustic environment, and their mechanisms are still poorly understood. In this study, ferrets were trained on a two-alternative forced-choice task to discriminate the azimuthal direction of brief sounds. In one experiment, pairs of noise bursts were presented from two loudspeakers at different interstimulus delays (ISDs). Results showed that localization performance changed as a function of ISD in a manner consistent with the PE being operative. A second experiment investigated buildup and breakdown of the PE by measuring the ability of ferrets to discriminate the direction of a click pair following presentation of a conditioning train. Human listeners were also tested using this paradigm. In both species, performance was better when the test clicks and conditioning train had the same ISD but deteriorated following a switch in the direction of the leading and lagging sounds between the conditioning train and test clicks. These results suggest that ferrets, like humans, experience a buildup and breakdown of the PE.
I. INTRODUCTION
The precedence effect (PE) is thought to provide an important mechanism for enabling reliable localization of sound sources in the presence of competing sounds (see Gardner, 1968, for a historical background). The neural mechanisms responsible are not fully understood yet but appear to involve both the auditory periphery (Bianchi et al., 2013, and references therein) and the central auditory system (e.g., Fitzpatrick et al., 1999; Litovsky and Yin, 1998).
The PE is often tested by presenting two identical or very similar sounds from different locations with a short delay between them (Wallach et al., 1949; Haas, 1951). Distinct perceptions of the leading and lagging sounds occur depending on the delay; in particular, the sounds' perceived location changes (Haas, 1951) (for review, see Blauert, 1997; Litovsky et al., 1999). At very short delays, or no delay at all, summing localization is observed: Subjects hear a single fused sound image from an intermediate position between the original two sound sources, indicating that both the leading and the lagging sound contribute to the perceived location (e.g., Blauert, 1997; Keller and Takahashi, 1996; Tollin and Yin, 2003). At longer delays, the leading sound source dominates the perceived location of the sound pair. The lagging sound might be audible, especially as the interstimulus delay (ISD) is increased (e.g., Pecka et al., 2007; Yang and Grantham, 1997), but its location is also perceived to be that of the leading loudspeaker. At still longer delays, the lagging sound becomes localizable, i.e., the two sounds are heard as separate events from their actual sound source positions; echo threshold is reached.
The distinct perceptions of the location of the two sounds can be used to investigate, by measuring their behavioral performance, whether nonhuman species also experience the PE. If the PE occurs, localization of the leading source should be either unaffected by the presence of the lagging sound or impaired depending on the delay between them. Using sound localization tasks, the PE has been observed in several nonhuman species (e.g., owls: Keller and Takahashi, 1996; cats: Tollin and Yin, 2003; gerbils: Wolf et al., 2010). The aim of the first experiment in this study was to determine whether the PE can also be demonstrated in ferrets, which have become one of the principal animal models of spatial hearing and particularly for studying the development and plasticity of the neural mechanisms involved (King et al., 2007; King et al., 2011). We used a left/right discrimination task to measure the PE in both ferrets and humans so that the performance of the two species could be compared directly.
The strength of the PE has been shown to depend on the listener's prior exposure to information about the leading and lagging stimuli (Clifton, 1987; Thurlow and Parks, 1961). Having subjects listen to multiple presentations of leading-lagging stimuli, at an ISD just above the localization dominance range, causes the lagging sound to “fade away.” This is known as buildup of precedence. Consequently, the echo threshold is raised relative to trials on which paired sounds are presented in isolation (Freyman et al., 1991; Yang and Grantham, 1997). The opposite effect, enhanced perception of the lagging sound, or reduced echo threshold, occurs if the sounds preceding the test pair lack coherence with the test sounds (Clifton and Freyman, 1989; Clifton, 1987; Clifton et al., 1994; Freyman and Keen, 2006; Freyman et al., 1991; Keen and Freyman, 2009). This is known as breakdown of the PE (Clifton, 1987), although other authors argue that it more accurately reflects buildup that has yet to occur (Blauert and Braasch, 2005; Djelani and Blauert, 2000, 2001) because the change between preceding sounds and test sound is thought to trigger adaptation to a new auditory scene (Freyman and Keen, 2006; Keen and Freyman, 2009).
Investigation of the adaptive processes that drive the PE can ultimately bolster our understanding of how humans are able to function in complex listening environments. Establishing an animal model that exhibits buildup and breakdown of the PE in its behavioral responses is therefore an important step toward identifying the underlying neural basis for these adaptive phenomena. Such behavioral data should also help to improve neural models of the PE that currently exclude buildup and breakdown (e.g., Xia et al., 2010). To date, only two studies have provided evidence that nonhuman species might experience buildup of the PE (Dent and Dooling, 2003; Kalmykova, 1993). In the second experiment, we therefore looked for evidence of buildup and breakdown by measuring the ability of ferrets to discriminate the direction of a leading-lagging pair presented after a train of conditioning sounds.
II. METHODS
A. Ferret psychophysics
1. Subjects
Eight adult pigmented ferrets (Mustela putorius furo, three males) were used in this study, of which three were trained with the entire stimulus set, whereas the others were tested in some aspects of the study only. The animals were housed (in pairs, if female) in standard laboratory cages with free access to dry food, water, and toys and allowed to interact with other ferrets on the floor of the room at least twice per week. During training, access to water was restricted from the day before the first testing session until after the last testing session of the training run. Training runs lasted between 3 and 14 days, with two to three training sessions per day. They were followed by breaks of at least 2 days. Ferrets received water as a positive reinforcement while performing a two-alternative forced-choice task to discriminate the direction of brief sounds as either coming from the left or from the right. During training, both the animals' water intake and their weights were monitored on a daily basis. Their water intake was supplemented as wet food (ground dry food mixed with water) up to a daily amount of 60 ml of water per kilogram of bodyweight. Additional wet food and a high calorie diet were provided if a weight drop persisted for more than two consecutive days during a training run.
All experimental procedures were approved by the local ethical review committees and carried out under license from the U.K. Home Office in accordance with the Animals (Scientific Procedures) Act 1986.
2. Training apparatus
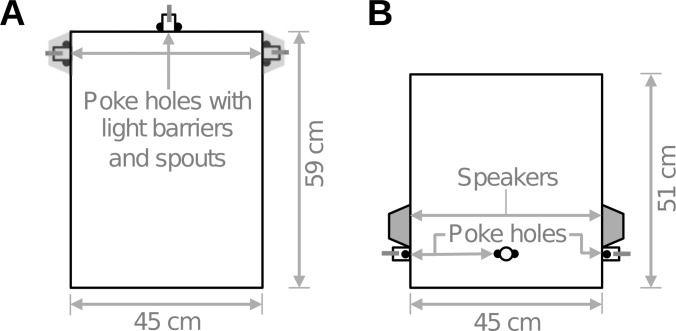
Animals were tested in an arena (inner dimensions: 51 cm × 45 cm × 59 cm, Fig. 1) comprising mesh walls and ceiling (10 mm opening) that was placed in a sound-attenuated booth lined with acoustic foam (MelaTech; Hodgson & Hodgson Ltd., Melton Mowbray, UK). The testing arena was fitted with three poke-holes (3 cm diameter), one in the center of the front wall and one at each side. Each poke-hole contained a photodiode and an infrared light emitting diode (LED) functioning as a light barrier that the ferrets had to break with their snouts to initiate a trial or respond to a stimulus. Water release from spouts inside the poke-holes was controlled by solenoids positioned outside the booth. Two loudspeakers (FRS 10; Visaton, Haan, Germany) were mounted at ±90° above the lateral poke-holes at a distance of 23 cm from the central poke-hole. Their outputs were flattened from 0.5 to 10 kHz using their finite impulse response filters. The behavioral task, data acquisition, and stimulus generation were all automated using a matlab-based interface and a TDT RP2.1 real-time processor running at 24.4 kHz (Tucker-Davis Technologies, Alachua, FL).
FIG. 1.
Schematic of arena used for behavioral testing, shown from above (A) and from the front (B). Ferrets initiated trials at the central poke-hole, which triggered the presentation of stimuli from speakers positioned above the poke-holes on the left and on the right. Light barriers in poke-holes on either side registered the ferrets' responses. On rewarded trials, ferrets received water rewards via spouts secured in the poke-holes.
3. Stimuli
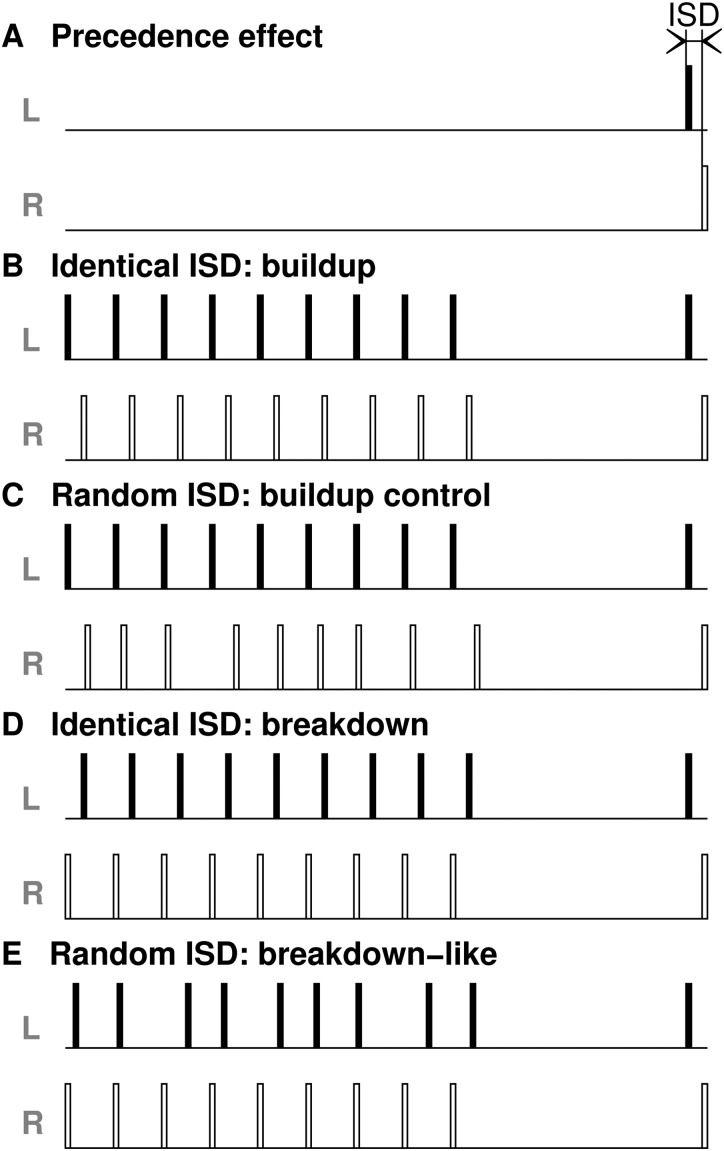
Stimuli were either broadband noise bursts of different durations with 1 ms cosine ramps, presented at a mean level of 68 dB sound pressure level (SPL) and roved by ±6 dB in 3-dB steps, or clicks comprising three sampling points (123 μs). Stimuli were presented at a peak-equivalent level of 66 dB SPL (roved by ±6 dB in 3-dB steps), either as single source sounds, from the left or right loudspeaker, or as paired source sounds from both loudspeakers with an ISD between the leading and lagging sound [Fig. 2(A)]. Negative ISDs denote conditions in which the left loudspeaker emitted the leading sound. In experiments investigating buildup/breakdown of the PE, animals were trained to respond to the target stimulus, which was either a single sound or a paired sound. The target was preceded by a conditioning train containing nine stimuli (paired or single sounds) presented at a repetition rate of 8 Hz and followed by a silent period of 750 ms [Figs. 2(B)–2(E)]. If clicks were used, the target stimulus comprised two (paired or single) clicks presented at a repetition rate of 3 Hz. In the case of noise bursts, just one target stimulus (paired or single noise burst) was presented. Noise tokens were identical within a trial but changed within a session from trial to trial.
FIG. 2.
Schematic of the stimulation conditions for paired source sounds, illustrated here for left-leading target sounds. (A) We tested for the presence of the PE using a pair of sounds with an interstimulus delay (ISD) between them. One of the sounds was delivered from the left (L) loudspeaker, whereas the other came from the right (R) loudspeaker. (B) In the buildup condition, the target stimulus was preceded by a left-leading conditioning train containing nine stimulus pairs with the same |ISD| as the target pair, followed by a period of silence. (C) In the condition used as a control for the buildup condition, the nine left-leading stimulus pairs had ISDs randomly chosen from a pre-determined set that excluded the target pair's ISD. (D) In the breakdown condition, the conditioning train was right-leading but with the same |ISD| as the left-leading target sound pair. (E) In the condition used to balance the presentation of paired source trials, the right-leading stimulus pairs had ISDs selected randomly from a set of values that excluded that of the target pair. The same applied for right-leading target sound pairs with the order of the paired sounds reversed accordingly.
4. Behavioral measurements of the PE
All eight ferrets were trained on the task investigating the PE, although not necessarily on both final target sound durations (5 ms noise bursts and 123 μs clicks).
A. Initial training.
During the first training session, which was carried out in the absence of any acoustic stimuli and lasted about 15 min, ferrets were trained to trigger water rewards from each of the spouts. Continuous noise was next presented from either side after the central spout was triggered, so that the animals learned to associate the sound source with a water reward (150 μl per trial) and to train them to return to the center poke-hole after approaching one of the reward spouts. Those early training sessions took approximately 20 min each. The initial training stage included further sessions in which the waiting time at the central poke-hole was steadily increased, the probability of receiving a water reward from the center spout was decreased, and the duration of the noise bursts was gradually reduced to 5 ms. The animals were trained to wait at the central poke-hole for 1–1.5 s (randomly varying) prior to sound presentation, with the reward probability at the center spout set to 0.05, until scores of ≥90% correct were obtained for 5 ms noise bursts. Typically, this took 30–40 sessions, each lasting 20–40 min.
B. Data collection.
A data collection run typically started with the presentation of long-duration noise bursts (≥100 ms), to encourage the animals to readily perform the task. The duration was reduced over three to five sessions to 5 ms or further to 123 μs. Animals were run on additional sessions with these short-duration sounds until they reached scores of >90% and >80% for 5 ms and 123 μs sounds, respectively. Paired source presentations were introduced only after these scores were achieved and were then randomly interspersed with single source trials, making up, on average, just 13% of all trials. The animals were rewarded on all paired source trials in which stimuli with a non-zero ISD were presented, irrespective of which reward spout they approached. The percentage of these trials therefore needed to be kept low to avoid satiating the animals or discouraging them from trying to localize the perceived sound source. Trials with an ISD of 0 ms were rewarded with a probability of 0.5 on the first presentation and with a probability of 1 after this, again to avoid reinforcing behavior, such as responding to one side only, for sounds that are presumably perceived as coming from the midline. ISDs of 0, 0.2, 0.6, 2, 5, and 10 ms were tested. Additional ISDs of 0.12, 1, and 20 ms were included for some ferrets. Each ISD was presented ≥30 times in seven ferrets; in one ferret, ISDs were each presented ≥15 times.
5. Behavioral measurements of the buildup/breakdown of the PE
Three ferrets were trained on the task investigating buildup and breakdown of the PE. All of them first completed runs during which data investigating the PE were collected.
A. Initial training.
Training for the buildup/breakdown paradigm started with an increase of the waiting time at the central poke-hole to 1.8 s. To facilitate learning, long duration (100 ms) noise bursts were used as target sounds. If animals waited reliably at the central poke-hole, a conditioning train was introduced, starting with a level close to the noise floor of the sound-attenuated booth. The level was gradually increased if the number of early alarms, i.e., the number of trials on which the animal left the central poke-hole before presentation of the target sound, did not exceed one-third of the total number of trials. Both the target stimulus and the sounds in the conditioning train were single source sounds of the same duration (5 or 20 ms at that stage of training). Animals were rewarded for approaching the side from which the target sound was emitted. Four possible combinations of conditioning train and target sound were presented: (1) A conditioning train from the left followed by a target sound from the left, (2) a conditioning train from the right followed by a target sound from the right, (3) a conditioning train from the left followed by a target sound from the right, and (4) a conditioning train from the right followed by a target sound from the left. Data collection commenced after levels for the conditioning train and the target sound were within 6 dB and animals scored reliably at ≥80% correct for target sounds (sound duration 123 μs at that stage of training). Ferrets took ∼30–40 sessions to complete the initial training stage.
B. Data collection for buildup and breakdown.
Each run started with presentation of long-duration noise bursts (100 ms) as target sounds and clicks in the conditioning train. The duration of the target sound was reduced over approximately five sessions to 123 μs, thus matching the duration of the sounds in the conditioning train. Animals were run on further sessions with these short-duration sounds until they reached scores of ≥80% for the target sound. No paired source stimuli were presented during those sessions.
Buildup and breakdown were tested by presenting paired source sounds, on average, in 13% of trials, randomly interspersed with single source sound trials. Paired source trials were rewarded irrespective of the animals' responses. As with the single source trials, conditioning trains and target stimuli comprising paired source sounds were presented in four combinations: (1) A left-leading conditioning train followed by a left-leading target sound, (2) a right-leading conditioning train followed by a right-leading target, (3) a left-leading conditioning train followed by a right-leading target, and (4) a right-leading conditioning train followed by a left-leading target. Conditions (1) and (2) tested the buildup, whereas conditions (3) and (4) tested the breakdown of the PE. The |ISDs| of the stimuli in the conditioning train and of the target sound were identical in both the buildup [Fig. 2(B)] and breakdown conditions [Fig. 2(D)].
In human psychophysical studies, the occurrence of buildup or breakdown is usually judged by comparing the performance of subjects on trials where the paired sound is presented in isolation with that on trials in which the paired sound is preceded by a conditioning train. However, we wanted to control for any changes in the level of attention or motivation that might have arisen from differences in the number of (any) sounds presented before the target sound by keeping the number of sounds in the conditioning trains constant throughout. In the control condition, it was necessary that the conditioning train had no effect on the perceptibility of the lagging sound in the target sound pair. This is not the case for single source sounds preceding a paired target sound (Freyman et al., 1991) or for repeated presentation of paired source sounds with a fixed ISD that is either the same (Clifton and Freyman, 1989; Freyman et al., 1991) or different from that of the target sound pair (Clifton et al., 1994).
The following condition was therefore chosen as a control for the buildup condition: Target sounds identical to those in the buildup trials were preceded by conditioning trains with stimulus pairs the |ISDs| of which were randomly chosen for each pair [Fig. 2(C)]. Those ISDs included the values tested in each ferret in the buildup/breakdown trials (see following text) and an additional set of ISDs common to all three animals tested: 0.2, 0.6, 2, 5, and 8 ms. The order of the sounds in the conditioning train and the target sound pair was identical to the combinations used in the buildup paradigm (see preceding text). The left/right discrimination performance for this control condition should be reduced compared to the buildup condition because the random |ISDs| in the conditioning train would be expected to cause the lagging sound to interfere with the localization of the leading sound. To balance the presentation of paired source sounds, target sounds preceded by a conditioning train with random, sign-inverted ISDs were also presented with the same probability during sessions [Fig. 2(E)].
To observe a breakdown of precedence, buildup has to occur first (Clifton and Freyman, 1989; Thurlow and Parks, 1961). Therefore the occurrence of breakdown was judged by comparing the animals' performance on the breakdown condition [Fig. 2(D)] with that on the buildup condition [Fig. 2(B)]. Localization performance for the target paired source sounds should be lower in the former due to the lagging sound interfering with the localization of the leading sound.
The |ISDs| tested for the presence of buildup and breakdown were chosen individually for each ferret based on its performance in the PE experiments. The choice of |ISD| was guided by the difference in performance between left-leading and right-leading paired source sounds, the so-called localization accuracy (see following text). |ISDs| were chosen so that they fell on the steep portion of the falling slope of the localization accuracy function. In two ferrets, all ISDs were tested ≥30 times for each condition; in one ferret, ≥20 trials were performed for each ISD and condition.
6. Localization accuracy
For some ISD values, both the leading and lagging loudspeakers were potentially localizable. In those cases, animals might approach each loudspeaker at chance level or develop a bias toward one of them. In the latter case, averaging across sides to maximize the number of trials per |ISD| would result in high scores that are misleading. The ferrets' performance was therefore measured as localization accuracy, which was calculated by subtracting the percentage of “right loudspeaker approached” trials at the negative ISD from the corresponding value at the positive ISD [Fig. 3(B)]. Localization accuracy can assume values between −100% and 100%, where a value of 100% indicates perfect performance with the animal approaching exclusively the leading loudspeaker for a given |ISD|, values close to zero indicate chance performance or a bias toward one of the two loudspeakers, and negative values result from a preference for the lagging loudspeaker.
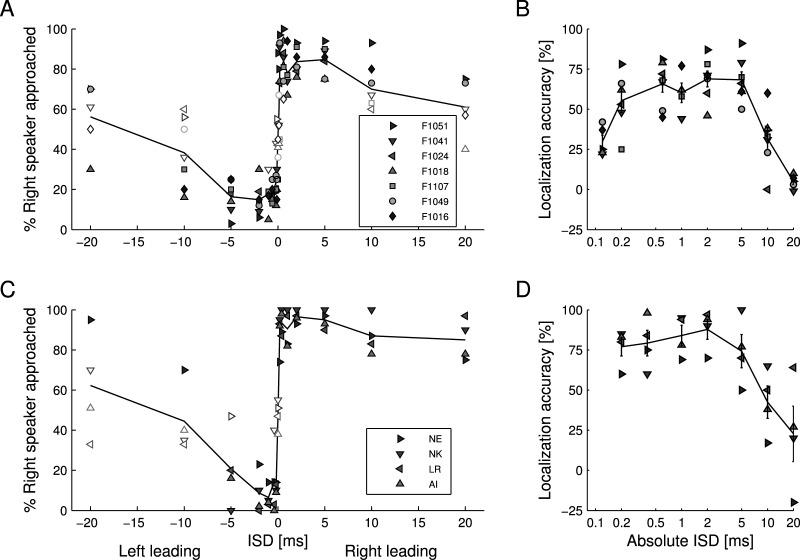
FIG. 3.
The PE in ferrets and humans. (A) Ferrets' performance on a left/right discrimination task for paired source sounds (duration, 5 ms) shown by plotting the percentage of trials in which the right loudspeaker was approached as a function of the ISD. Individual data from seven ferrets and their mean performance are shown by the symbols and line, respectively. Open symbols represent data that are not significantly different from chance performance (two-way binomial test tested at p = 0.05). Triangles indicate animals (n = 4) that were first (or only) tested with 5 ms noise bursts. All other animals (n = 3) were first tested with clicks. (B) Ferrets' localization accuracy depends on |ISD|. This was calculated as the difference between the percentage of trials in which the right loudspeaker was approached at the negative ISD and the corresponding value at the positive ISD. Larger values indicate that the animals were more likely to approach the source of the leading sound. (C) Performance of four human subjects tested on a left/right discrimination task for paired source sounds of 5 ms duration. Individual and mean performances are shown as symbols and line, respectively. Open symbols again represent data that are not significantly different from chance performance. (D) Human subjects' localization accuracy for the data shown in (C). For clarity, the range of localization accuracy is limited to values above −25%.
7. Reaction times
Reaction time denotes the time interval between the onset of the target stimulus and the time point at which the animal withdrew its head from the central poke-hole, re-establishing the light barrier. Trials with reaction times of <100 ms and >3 s were regarded as early alarms and missed trials, respectively, and excluded from the analysis. The distribution of reaction times can be described by an ex-Gaussian distribution (Matzke and Wagenmakers, 2009) [Fig. 4(A)]. We therefore report reaction times of individual animals as median and interquartile range. Statistical testing of the raw data was carried out using nonparametric tests, including the Mann-Whitney U test and the Kruskal-Wallis one-way analysis of variance.
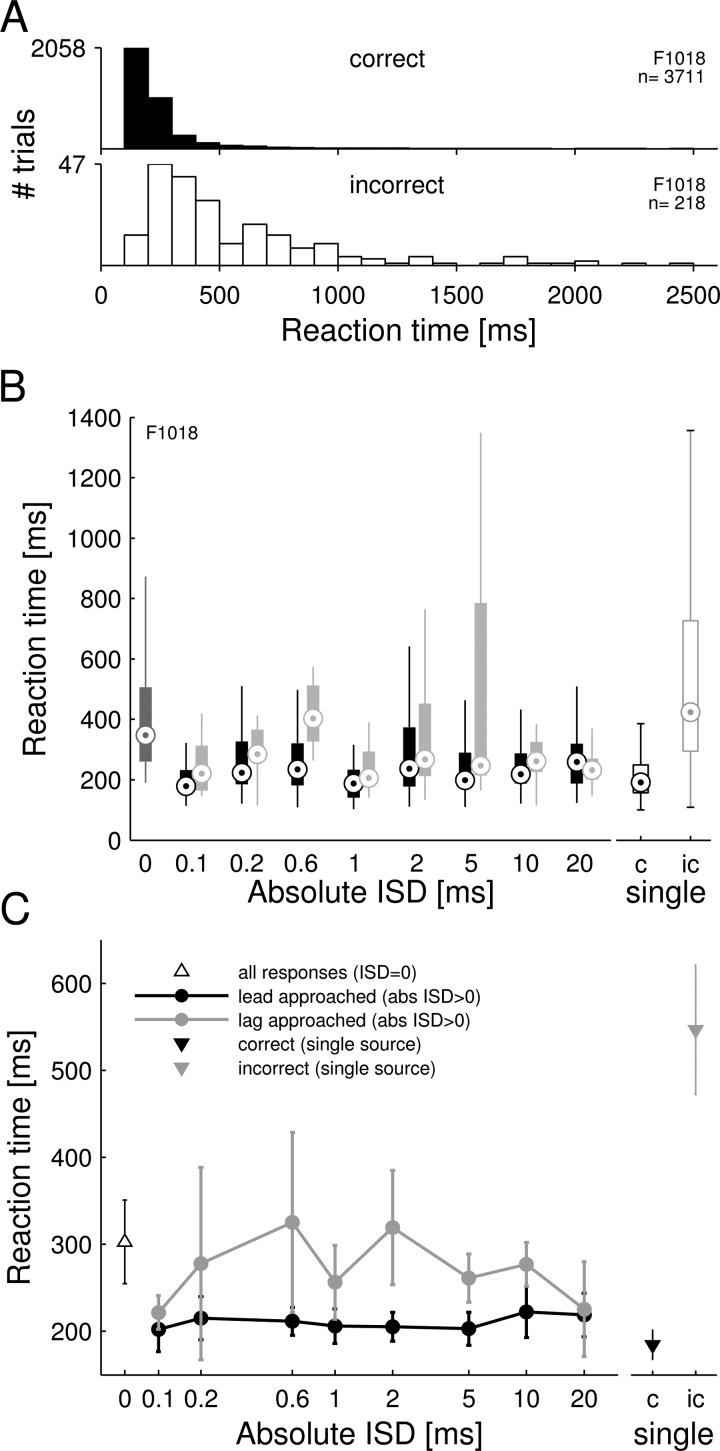
FIG. 4.
(A) Distribution of reaction times for correct (upper panel) and incorrect (lower panel) single source trials of one animal (F1018) (bin size 100 ms). Reaction time was defined as the time measured between the onset of the target sound and the animal's withdrawal from the central waterspout. (B) Reaction times for paired source trials when this same animal (F1018) approached either the leading loudspeaker (black) or the lagging loudspeaker (gray). Median values for trials on which the lagging loudspeaker was approached were significantly higher than for trials on which the leading loudspeaker was approached (p = 0.00028, Mann-Whitney U test). Reaction times for |ISD| = 0 are shown on the left of the figure (dark gray boxplot). For comparison, reaction times for correct (c) and incorrect (ic) single source trials are shown on the right (open boxplots). (C) Median reaction times averaged for all seven ferrets. Reaction times for trials on which the lagging loudspeaker was approached (gray) were longer than those for trials on which the leading loudspeaker was approached (black). Mean values for correct (black filled triangle) and incorrect (gray triangle) single source trials and for paired source trials with |ISD| = 0 (open triangle) are shown for comparison.
B. Human psychophysics
Four human subjects (3 male, 1 female, ages: 32–35 yr) were tested. Human psychophysical procedures were carried out under the guidelines of the Central University Research Ethics Committee of the University of Oxford. An attempt was made to make the task as similar as possible to those performed by the ferrets.
Subjects were seated in a double-walled sound-attenuating chamber halfway between a pair of loudspeakers (FRS 10; Visaton, Haan, Germany), separated by 120 cm, at ±90° and at ear height (105 cm). Subjects used a chin rest to minimize/prevent head movements. They were able to monitor their progress in each session on a flat-screen monitor and indicated the perceived direction of the target sound by pressing keys on a keyboard. If they heard two sounds, subjects were instructed to indicate the direction of the more salient one. The target sound was presented either after a short period of silence (500 ms) to investigate the PE or after a conditioning train and a period of silence (750 ms) to test for buildup/breakdown.
In contrast to the procedure used with the ferrets, all trials were paired source trials. Feedback was not given beyond an initial short session to familiarize subjects with the behavioral setup and the procedure. There were also no correction trials, and stimuli were exclusively 5 ms noise bursts. |ISDs| tested in the sessions investigating the PE were 0, 0.2, 0.4, 1, 2, 5, 10, and 20 ms. Two subjects were additionally tested on an ISD of 0.12 ms. In three subjects, 30 trials/ISD (and in one subject, 20 trials/ISD) were collected in a single session lasting ∼30 min. As with the ferrets, the |ISDs| used in the sessions investigating buildup and breakdown were derived from the PE data of each subject. Values were chosen so that they corresponded to localization accuracy values on the falling slope of the function (Fig. 6). Data were collected in two to three sessions with a total duration of ∼90 min. In three subjects, 15 trials/ISD (and in one subject, 30 trials/ISD) were collected for these buildup/breakdown experiments. All other aspects of the testing procedure, stimulus generation, and stimulus configurations were identical to those used with the ferrets.
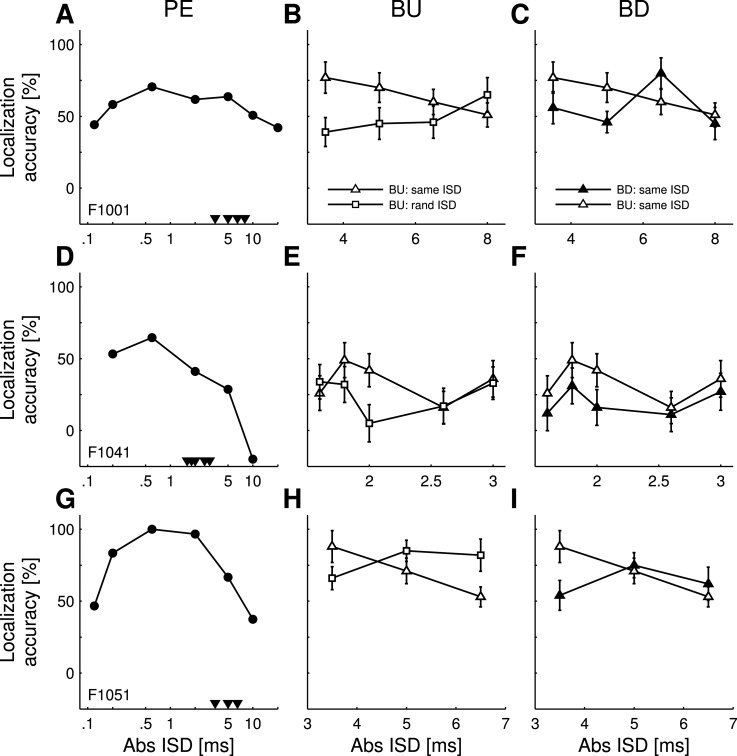
FIG. 6.
Four human subjects (rows) were tested for the PE (left column) and the BU (middle and right column) and BD (right column) of the PE. Symbols as in Fig. 5.
C. Statistical analysis
Data are presented as means and standard error of the mean (SEM). Medians and quartiles are reported if the data did not pass the Lilliefors test. The degree to which the responses were dominated by the leading loudspeaker in the PE experiments was assessed using the two-way binomial test at a significance level of p = 0.05. Significance in the buildup/breakdown experiment was assessed by bootstrapping: The localization accuracy was calculated from 1000 re-samples of the original data from each subject. The standard deviation of the localization accuracy estimated that way therefore corresponds to the SEM. Data analysis was carried out using matlab software (The Mathworks, Inc., Natwick, MA) and IBM SPSS 21.0 Statistics.
III. RESULTS
A. Ferret psychophysics
1. Ferrets experience the PE
Seven ferrets were tested for the PE with noise bursts (duration 5 ms) using a left/right discrimination task that was designed so that their responses were based on the perceived location of the paired target sound. Single source sounds, which accounted for ∼87% of trials, were correctly localized on >90% (mean ± SD: 95% ± 2%) of trials with individual scores varying from 93% to 98%. Performance for the randomly interleaved paired source sounds depended on the |ISD| [Fig. 3(A)]. At an ISD of 0 ms, ferrets approached the right loudspeaker, on average, in 55% ± 20% of trials. This was not significantly different from chance performance (two-way binomial test, p = 0.05), suggesting that stimuli presented simultaneously from both loudspeakers were perceived as coming from the midline.
At |ISDs| from 0.2 to 5 ms, ferrets approached the loudspeaker emitting the leading sound at a high percentage and significantly above chance [Fig. 3(A)]. This is in accordance with the perceived location of such paired source sounds being dominated by the leading sound. To define the lower boundary at which localization dominance was observed, five animals were tested at an |ISD| = 0.12 ms. They exhibited a preference for one of the loudspeakers, irrespective of whether it was the source of the leading or lagging sound, suggesting a failure of localization dominance. At |ISDs| ≥ 10 ms, most animals again did not reliably approach the leading loudspeaker. Either their performance for both left-leading and right-leading sounds dropped to chance performance or they developed a bias toward one of the two loudspeakers.
A. Effect of ISD and leading side.
A two-way repeated measures analysis of variance (ANOVA) with ISD and leading side as factors was run on the data from four ferrets that were tested for the full range of ISDs. This showed that ISD had a significant effect on performance [F(2.338,7.013) = 19.774; p < 0.001; partial η2 = 0.868]. The leading side (left or right) was not significant [F(1,3) = 0.494; p = 0.533; partial η2 = 0.141] and neither was the interaction between ISD and leading side [F(1.714,5.142) = 1.231; p = 0.336; partial η2 = 0.291].
B. Localization accuracy.
To better visualize the range over which localization dominance is likely to operate, we calculated the localization accuracy for each |ISD| by subtracting the percentage “right loudspeaker approached” for the negative ISD from the corresponding value for the positive ISD [Fig. 3(B)]. That way, chance performance values and bias toward one loudspeaker translated into small localization accuracy values. For |ISDs| from 0.2 to 5 ms, the average localization accuracy was >50%. It decreased sharply for higher and lower values, i.e., at |ISDs| ≥ 10 ms and at an |ISD| = 0.12 ms, suggesting that localization dominance failed at these ISDs.
C. Reaction times.
Each animal's reaction times were measured on all trials. For single source trials, all animals had significantly longer reaction times when they approached the wrong spout than when they responded correctly (p < 0.001, Mann-Whitney U test). Averaging the median reaction times across all animals gave mean ± SD values of 184 ± 44 ms on correct trials and 547 ± 196 ms on incorrect trials. The distributions of single-source reaction times for a single animal are shown in Fig. 4(A). The medians for those distributions, and the average medians across all animals, are plotted on the right side of Figs. 4(B) and 4(C), respectively.
We observed a similar effect in paired source trials in which approaching the lagging and leading loudspeakers can be regarded as incorrect and correct, respectively [Figs. 4(B) and 4(C)]. For the majority of |ISDs| tested, reaction times were longer on trials in which the animals approached the lagging loudspeaker (mean ± SD of the median reaction times across all animals and |ISDs| = 284 ± 102 ms) than on trials in which the animals approached the leading loudspeaker (212 ± 52 ms). In all animals, this difference was significant (p < 0.001, Mann-Whitney U test). For an ISD of 0 ms, the average median reaction time across all animals was 303 ± 127 ms; an arbitrary subdivision of these data according to whether the right or left loudspeaker was approached did not yield any significant differences.
Even though the categorization of paired source responses into correct and incorrect responses is reflected in the reaction times, human studies have shown that the percept of paired source sounds differs from that associated with single source sounds (Blauert and Braasch, 2005; Haas, 1951). In all animals, the reaction times for paired source trials were longer than those for correct single source trials, suggesting a decrease in decision confidence (p < 0.001 for all animals, Mann-Whitney U test) [Fig. 4(C)]. Interestingly, the reaction times measured for trials on which the animals approached the lagging loudspeaker were much shorter than those measured on incorrect single source trials.
Because the percept of paired source sounds changes depending on the |ISD|, we might expect reaction times to do so as well. However, we found no significant differences across |ISD|, either for trials on which the animals approached the leading loudspeaker [ANOVA F(8,46) = 1.41, p = 0.219] or for trials on which they approached the lagging loudspeaker [F(8,46) = 1, p = 0.45] [Figs. 4(B) and 4(C)].
2. The PE for clicks in ferrets
The PE is often tested with much shorter sounds than the ones used in the experiment described in the preceding text. We therefore tested six ferrets with clicks (123 μs), instead of 5 ms noise bursts, to allow for a more direct comparison. The ferrets' localization performance again changed with |ISD| [three examples in Figs. 5(A), 5(D), and 5(G), left column]. A two-way repeated measures ANOVA with ISD and leading side as factors showed that ISD had a significant effect on performance [F(4,20) = 10.65; p < 0.001; η2= 0.681]. Leading side was not significant [F(1,5) = 0.206; p = 0.669; partial η2 = 0.04] and neither was the interaction between ISD and leading side [F(4,20) = 0.11; p = 0.978; partial η2 = 0.022]. As with the longer duration sounds, the |ISD| range over which the mean localization accuracy was >50% extended from 0.2 to 5 ms. However, variability was higher across individuals, resulting in more shallow slopes and a less well-defined range in the population data (not shown) at the |ISDs| where localization dominance is likely to operate. Localization accuracy values for clicks presented with |ISDs| ≥ 5 ms were smaller than those calculated for 5 ms noise bursts. For an |ISD| = 10 ms, the mean localization accuracy across animals was only 15% (compared to 30% for the longer duration sounds). Notably, the smaller localization accuracy values were not reflected in the scores for the single source sounds, indicating equivalent performance for such short sounds when presented in isolation. The median score averaged across animals for approaching the correct side was 96% ± 2%, with the medians varying between individuals from 93% to 98%.
FIG. 5.
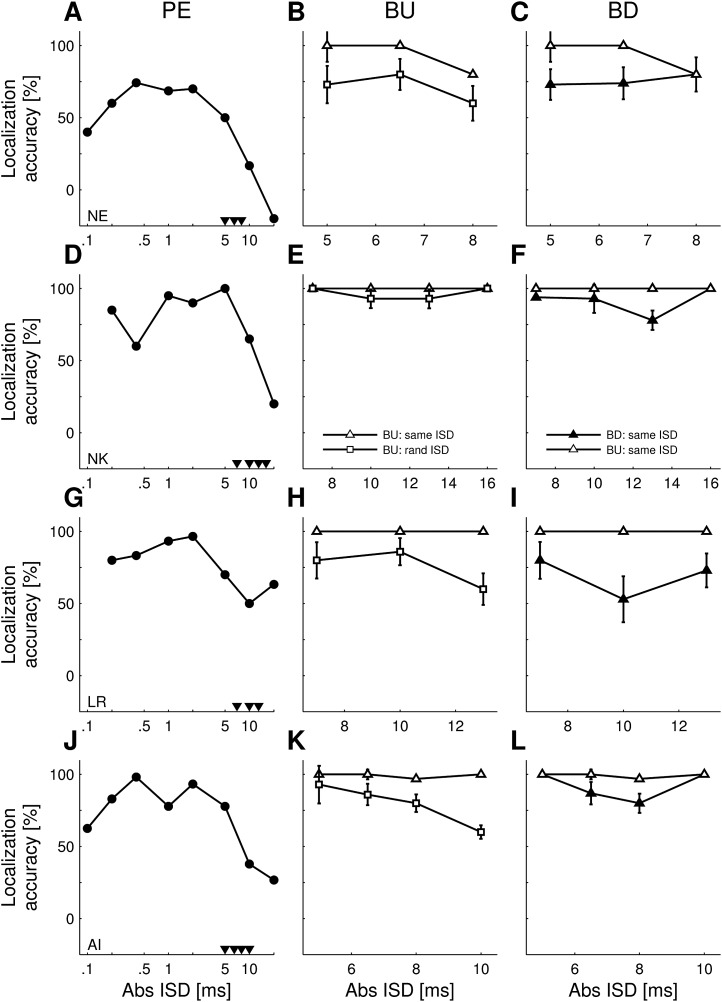
Three ferrets (rows) were tested for the PE (left column) as well as its buildup (BU, middle and right column) and breakdown (BD, right column). Arrowheads in the left column mark the |ISDs| that were tested in the experiment investigating BU/BD. In some trials, the target sound pair was preceded by a conditioning train of nine paired sounds with either the same (“BU: same ISD”) or the same but sign-inverted (“BD: same ISD”) ISD as in the target sound pair (triangles). In other, control trials, the target sound pair was preceded by nine paired sounds in the same lead/lag presentation order but with random ISDs that excluded that of the target sound pair (squares; “BU: rand ISD”). Error bars show the SEM derived from bootstrapping by calculating the localization accuracy from 1000 resamples of the original responses.
3. Buildup and breakdown of precedence in ferrets
Three ferrets were tested for buildup/breakdown of the PE. Paired source trials (i.e., buildup trials, breakdown trials, and control condition trials) were randomly interleaved with single source trials and made up ∼13% of trials per session. For this experiment, the stimuli used were 123 μs clicks. |ISDs| were chosen individually for each ferret from the steepest slope region of the localization accuracy function (Fig. 5, arrowheads in left column). |ISDs| from 1.6 to 8 ms were tested, corresponding to localization accuracy values between 37% and 82%.
Ferrets' scores for single source versions of the buildup/breakdown trials indicated that the animals were able to do the task, i.e., waiting throughout a conditioning train and then responding to the direction of the following target sound. Targets in buildup-like trials, where a conditioning train and target were presented from the same loudspeaker, were localized correctly in 88% ± 5% of trials. Targets in breakdown-like trials, where a conditioning train and target were presented from different loudspeakers, were also localized correctly in most (89% ± 4%) trials. Those scores correspond to average localization accuracy values of 74% ± 9% and 79% ± 9% for buildup-like and breakdown-like single source trials, respectively.
A. Buildup of precedence.
Buildup of the PE can be observed when a paired sound with an ISD just above the localization dominance range is repeated a number of times; compared to trials on which no repetitions of the paired sound are presented, subjects are less likely to perceive the lagging sound (Freyman et al., 1991; Thurlow and Parks, 1961). In addition to this buildup condition [Fig. 2(B)], we used a control condition that included the same number of paired sounds in the conditioning train, but the |ISDs| in each pair were chosen randomly from a set of values that excluded the |ISD| of the target sound [Fig. 2(C)]. Within each trial in the control condition, ISD values in the conditioning train and the target sound had the same sign. The performance, measured as the localization accuracy, was then contrasted with the performance in the buildup paradigm.
In all three ferrets, the scores for the buildup condition were significantly higher than those for the control condition at certain |ISDs| as demonstrated by the non-overlapping error bars in Figs. 5(B), 5(E), and 5(H). These data therefore suggest that ferrets experience a buildup of the PE. In buildup trials, localization accuracy tended to decline as the |ISD| was increased, whereas the reverse trend was observed for the control condition.
B. Breakdown of precedence.
After the PE has built up following repeated presentation of a paired sound with an ISD just above the localization dominance range, reversing the sign of the ISD, i.e., switching the leading/lagging loudspeaker positions, can result in subjects being able to once again localize the lagging sound (Clifton, 1987). To determine whether ferrets experience PE breakdown, identical paired sounds were presented throughout the conditioning train and target sound except that the sign of the ISD was switched between them [Fig. 2(D)]. The same |ISDs| used in the buildup trials were tested in each subject. Performance in this breakdown condition was then compared with the buildup condition [Figs. 5(C), 5(F), and 5(I)].
In all ferrets, localization accuracy values for the breakdown condition were significantly lower than those for the buildup condition at certain |ISDs|. For the remaining |ISDs|, localization accuracy did not differ significantly between these two conditions. However, scores in the breakdown condition tended to be lower than in the buildup condition, suggesting that the lagging sound had a greater influence on the animals' localization behavior in the breakdown trials [Figs. 5(C), 5(F), and 5(I)].
B. Human psychophysics
1. The PE in humans
Four subjects participated in a left/right discrimination task to test for the PE. Overall, they showed greater localization accuracy than the ferrets. As with the ferrets, humans showed summing localization at very short |ISDs|, suppression of the lagging sound at slightly longer |ISDs|, where the perception of sound direction was dominated by the leading sound, and a failure of localization dominance at longer |ISDs| [Figs. 3(C) and 3(D)]. Summing localization in the data was most apparent for an ISD of 0, where the subjects reported that the sounds originated at the center of the frontal hemifield. At this ISD, the subjects gave a right response on an average of 42% ± 20% of trials. Two subjects were additionally tested using paired sounds with an |ISD| of 0.12 ms [Figs. 6(A) and 6(J)]. They showed localization accuracy values of 40% and 60%, supporting the evidence that summing localization is operating at small ISDs. As in the ferrets, the highest localization accuracy values were obtained at |ISDs| from 0.2 to 5 ms, while the lowest values were found for |ISDs| ≥ 10 ms. At these longer ISDs, subjects reported perceiving both the leading and the lagging sound.
A. Effect of ISD and leading side.
A two-way repeated measures ANOVA with factors leading side and ISD showed that performance was dependent on ISD [F(2.174,6.522) = 10.945; p < 0.001; η2 = 0.785]. There was a non-significant trend for leading side to affect performance [F(1,3) = 8.8; p = 0.059; partial η2 = 0.746]. The partial η2, a measure of effect size, revealed that ∼75% of the total variance of the data could be explained by differences in leading side. Detecting a significant difference at the significance level of 0.05 was likely impeded by the small sample size (n = 4). There was a significant interaction between ISD and leading side [F(1.964,5.891) = 6.841; p = 0.001; partial η2 = 0.695].
2. Buildup and breakdown of precedence in humans
A. Buildup of precedence.
To determine whether humans' reported ability to experience buildup of the PE (e.g., Freyman et al., 1991; Yang and Grantham, 1997) can be tested in a left/right spatial discrimination task, the same four subjects participated in a second experiment designed to match that used with the ferrets. We compared the performance of these subjects in buildup trials with that in control trials, where the |ISDs| of the sound pairs in the conditioning train changed randomly for each sound pair, but excluded the target sound pair's |ISD|. As in the ferrets' task, only |ISDs| just above the range of localization dominance were tested. They were chosen individually for each subject from within the falling slope of the localization accuracy functions obtained in the PE experiment (Fig. 6, arrowheads in left column). These values ranged from 5 to 16 ms and corresponded to localization accuracy values between 30% and 86% and were therefore in the same range tested in the ferrets.
Three of four subjects showed a range of |ISDs| where localization accuracy was significantly higher in the buildup condition than in the control condition, as indicated by the non-overlapping error bars [Figs. 6(B), 6(E), 6(H), and 6(K)], indicating that, as in ferrets, a left/right discrimination task can be used to demonstrate buildup of localization dominance in the PE.
B. Breakdown of precedence.
Breakdown was tested in the same sessions as buildup with trials randomly interleaved. In contrast to the buildup paradigm, the sign of the ISD switched between the conditioning train and the target sound. The same |ISDs| as in the buildup trials were tested in each subject.
All four subjects showed |ISDs| where the breakdown paradigm yielded significantly lower localization accuracy values than the buildup condition, as indicated by the non-overlapping error bars [Figs. 6(C), 6(F), 6(I), and 6(L)]. These data therefore show the same pattern as in the ferrets and suggest that breakdown of the PE was achieved in each case.
IV. DISCUSSION
The PE is thought to contribute to the ability of listeners to localize sounds reliably in the presence of acoustic reflections. Potential confusion regarding the location of the source can be resolved either by integrating or disregarding competing information from echoes. Both peripheral mechanisms (Bianchi et al., 2013) and higher order processing in the auditory system (e.g. Backer et al., 2010; Damaschke et al., 2005) appear to contribute to the PE. Investigation of this phenomenon, and particularly its buildup and breakdown, which are likely to reflect central processing, should therefore provide valuable insights into the neural mechanisms involved in listening in complex acoustic environments. By presenting brief sounds from two different loudspeakers at a range of ISDs, we found that localization performance is affected in a similar way in ferrets and humans, suggesting that both species experience the PE. We also obtained evidence for buildup and breakdown of localization dominance in both species. These data show that the ferret is a suitable model animal for the investigation of the PE and, more generally, the investigation of listening in complex acoustic environments.
A. Ferrets experience the PE
Our findings add to a growing body of evidence that the PE occurs in nonhuman species (e.g., budgerigars, canaries, and zebra finches: Dent and Dooling, 2004; owls: Keller and Takahashi, 1996; cats: Tollin and Yin, 2003; gerbils: Wolf et al., 2010). Although the results of these studies are broadly consistent across species, the details vary according to the paradigm employed to measure the PE. In the present study, we were able to make a direct comparison between ferrets and humans by testing both species using the same stimuli and task. The shapes of the localization accuracy functions obtained in each species were very similar, indicating that ferrets likely experience the PE in a comparable way to humans.
One other study measured the PE of different species in the same task. Dent and Dooling (2004) reported that the time course of the PE is similar in three species of birds, although differences were found in their overall behavioral performance. Because these data were collected using the same apparatus and testing procedures, Dent and Dooling (2004) argued that the difference in scores reflects a difference in perceived sound quality between the species. Similarly, our results suggest that room echoes are likely to have a comparable impact on the localization performance of ferrets and humans given that the observed differences in localization accuracy between these species were marginal.
B. Measuring the PE with a left/right discrimination task
Our approach for measuring the PE was based on the assumption that paired source sounds provide localization cues that can be used in a left/right discrimination task. Previous work in which sound pairs were presented from two loudspeakers located at ±20° around the midline has shown that directional judgments by human listeners change with ISD (Perrott et al., 1987). Consistent with this, we found that left/right discrimination performance, measured as localization accuracy, varied with ISD. By not providing feedback on paired sound trials (ferrets were rewarded for responding to either loudspeaker location) and presenting these stimuli infrequently among single source sounds, our results should reflect the animals' spontaneous localization behavior.
Psychophysical studies in humans have shown that lag detection thresholds (“Did you hear one or two sounds?”) are lower than echo thresholds (“Did you perceive a second sound with a distinct location?”) (Pecka et al., 2007). We assumed that an ability to detect the lagging sound at ISDs where it cannot be localized should not affect localization of the leading sound in a pair. Human subjects were therefore instructed to indicate the location of the more salient sound if they perceived both sounds. In the present study, ISDs just above the localization dominance range most likely correspond to the echo threshold, i.e., where two sounds are perceived that originate from distinct sources. In both species, localization dominance failed at ISD > 5 ms. Thus if echo thresholds in ferrets and humans had been tested with a more conventional measure, they would probably lie between 5 and 10 ms for the 5 ms noise bursts used in the present study.
C. Left-right asymmetry when measuring the PE
In human subjects, we found that localization dominance was more prominent for paired sounds originating first from the right loudspeaker than from the left loudspeaker. Left-right asymmetries in the context of the PE have been reported in studies investigating buildup and breakdown but not when paired sounds were presented in isolation (Clifton and Freyman, 1989; Grantham, 1996). This led to the conclusion that the adaptive processes affecting the PE involve cortical processing (Grantham, 1996), a possibility supported by electrophysiological studies in humans (Dimitrijevic and Stapells, 2006; Spierer et al., 2009). Recently, as in the present study, asymmetric responses for paired sounds presented in isolation have been reported in a larger group of human subjects (Bishop et al., 2011), suggesting that procedural differences are unlikely to account for the difference from the earlier reports. Although a left-right asymmetry was not present in ferrets at the population level, two of the animals [F1051 and F1049, Fig. 3(A)] did show asymmetries similar to those observed in humans [Fig. 3(C)]. A statistical analysis of the effect of the leading side on buildup/breakdown of the PE was not run due to the limited dataset available, but this issue warrants further investigation, particularly at a neurophysiological level, because of its implications for the neural origins of the PE and its buildup/breakdown.
D. What can reaction times tell us about the animals' perception of paired source sounds?
In two-choice decision tasks, error responses are typically associated with elevated reaction times (Ratcliff and McKoon, 2008), which are thought to result from the extended period during which information is gathered before a response is made (Ratcliff, 1978). In keeping with this, we found that the ferrets' reaction times on single source trials were significantly longer when they approached the incorrect speaker than when they made a correct choice. Similar results have previously been reported when ferrets are engaged in a multiple speaker localization task (Nodal et al., 2008).
We separated the reaction times of paired source trials into trials in which the animals approached the leading sound and those in which the animals approached the lagging sound, on the assumption that, for a range of non-zero ISDs, “leading sound approached” corresponds to a correct response. As expected, reaction times were longer when the animals approached the lagging speaker than when they responded to the leading speaker. This suggests that, as in the single source trials in which response choices are more clearly delineated as correct and incorrect, paired source sounds induce different levels of decision confidence that are reflected in the way reaction times vary with the perceived location of the sounds.
The reaction times on paired source trials where the ferrets approached the leading sound were longer than those measured for correct responses on single source trials. This suggests that uncertainty is increased for paired source sounds, even for perceptually correct responses. In contrast, previous studies in cats found that the latency of saccadic eye and pinna movements did not differ between single and paired source trials for ISDs in the range of localization dominance (Tollin and Yin, 2003; Tollin et al., 2010).
We speculated that uncertainty, and therefore reaction time, would change as the ISD was varied with the lowest uncertainty and shortest reaction times occurring at ISDs in the localization dominance range, whereas decisions should be less certain when summing localization occurs at smaller ISDs. Our results confirmed this, as reaction times on trials with an ISD of 0 ms exceeded those of the other paired source conditions. This finding suggests a high level of uncertainty resulting from the lack of a clear spatial percept in either hemifield. At ISDs exceeding the localization dominance range, however, the drop in localization accuracy was not associated with longer reaction times, and, on average, the animals approached the leading and lagging sounds equally quickly.
E. Buildup and breakdown of the PE in ferrets
Buildup and breakdown of the PE are thought to reflect the auditory system's capacity to adapt to a listener's acoustic environment (Clifton and Freyman, 1989; Clifton et al., 1994; Keen and Freyman, 2009). Initially the soundscape, including direct sounds and their reflections, is analyzed with regard to the spatial dimensions of the listener's surroundings and the presence or absence of nearby objects. As a model of auditory space is established, indirect sounds are suppressed to facilitate the localization and identification of sound sources. Subsequent changes in the acoustic environment require a re-assessment of the sounds reaching the ears with direct sounds and their reflections being perceived once again by the listener before being suppressed upon the establishment of an adjusted model of auditory space.
To the best of our knowledge, only two studies have previously carried out a systematic investigation of the buildup of the PE in nonhuman animal species. Kalmykova (1993) found higher echo thresholds for trains of click pairs than for single click pairs in cats, where the task was to indicate the direction of the leading click in the pair. Dent and Dooling (2003) used a go/no-go task in which the ability of budgerigars to discriminate left-leading background stimuli from right-leading target stimuli (or vice versa) was tested and found that performance improved as the number of repetitions of the background stimuli was increased. These studies therefore suggest that buildup of the PE is found in species other than humans.
Here we investigated buildup and breakdown of the PE in ferrets and human subjects using the same paradigm, allowing for a direct comparison of the results in each species. Our data show that the buildup effect occurs in ferrets as well as humans, although some differences were observed. In humans, we found a buildup of localization dominance over almost the entire range of ISDs tested, whereas the range of ISDs where this occurred in ferrets appeared to be more limited. In both cases, we tested ISDs that lay on the steepest portion of the falling slope of the localization accuracy function. However, because the increase in echo threshold depends on the ISD tested (Freyman et al., 1991; Yang and Grantham, 1997), it is possible that we missed the lead-lag intervals that induce the greatest buildup in ferrets.
Buildup of the PE has been shown to depend on the number of stimulus repetitions (Clifton and Freyman, 1989; Freyman et al., 1991). In humans, the increase in echo threshold starts to asymptote after nine repetitions, suggesting that buildup may have saturated by then (Freyman et al., 1991). We therefore used nine stimulus repetitions in the preceding conditioning train when we tested both humans and ferrets. The dynamics of PE buildup are, however, not necessarily the same in different species. Indeed the other animal studies included conditions in which a larger number of repetitions were used (Dent and Dooling, 2003; Kalmykova, 1993), so it is also possible that including more stimulus presentations in the conditioning train would have produced a greater increase in localization accuracy in ferrets.
We examined whether breakdown of the PE occurs by comparing localization accuracy in the buildup and breakdown conditions. The rationale for this was that for breakdown to happen, it is necessary that a buildup of the PE occurred first. Most of the estimates of localization accuracy in ferrets and humans were higher for the buildup stimuli than for the breakdown stimuli, suggesting that breakdown of the PE occurred in both species.
In the present study, we used the term “breakdown” as coined by Clifton (1987) to refer to the increased perceptibility of the lagging sound following a reversal in the directions from which the leading and lagging sounds were presented. More recently, breakdown has been viewed as the onset of adaptation to a change in room acoustics (Freyman and Keen, 2006; Keen and Freyman, 2009) or buildup that has not occurred following that change (Blauert and Braasch, 2005; Djelani and Blauert, 2000, 2001). Recordings from neurons in the auditory pathway in an animal model should provide insights into the neural mechanisms underlying these poorly understood adaptive processes. Showing behaviorally that ferrets experience comparable buildup/breakdown of the PE is a first step toward realizing that.
F. Where does the buildup/breakdown of precedence originate?
The site of origin of buildup and breakdown of the PE within the auditory pathway is presently unknown. Although neural correlates of the PE have been reported at virtually all levels of the auditory system (e.g., Fitzpatrick et al., 1999), no evidence to date for buildup or breakdown has been observed in the responses of individual auditory neurons. Thus Fitzpatrick et al. (1999) measured the suppressive effect of a leading sound on neural responses to a lagging sound in individual trials and found no evidence for a buildup of precedence at any level of the auditory system investigated. Similarly, Litovsky and Yin (1998) found no changes in the responses of inferior colliculus neurons to the lagging sound during a 50-trial sequence of stimuli and again concluded that the buildup was not present in the responses of these neurons.
Studies investigating buildup and breakdown of precedence in humans are not clear about the site of origin either. On the one hand, electroencephalographic recordings suggest that buildup and breakdown involve higher order processing (Backer et al., 2010; Dimitrijevic and Stapells, 2006; Sanders et al., 2011). On the other hand, studies employing headphone stimulation found differences in buildup/breakdown depending on the localization cue used, suggesting a site of origin before information about interaural time and level differences is integrated (Brown and Stecker, 2013; Krumbholz and Nobbe, 2002; Spierer et al., 2009). Nevertheless, recent work in ferrets has revealed a greater capacity of neurons to adapt to sound statistics at higher levels of the auditory pathway (Rabinowitz et al., 2013). To the extent that buildup and breakdown of the PE reflect the auditory system's capacity to adapt to a listener's acoustic environment, we might therefore expect these phenomena to be observed in the response properties of cortical neurons.
G. Conclusions
The present study used the same stimuli and task design to show that the time course of the PE and its impact on localization accuracy are very similar in ferrets and humans. Moreover, differences in reaction times suggest that ferrets perceive single source sounds and paired source sounds differently. Both species show significantly higher scores in a left/right discrimination task if the target sound pair is preceded by a conditioning train comprising identical copies of this stimulus than if it is preceded by paired sounds of varying ISDs, indicating that a buildup of localization dominance can occur. If the location of the leading and lagging sounds is reversed between the conditioning train and the target sound pair, performance in the left/right discrimination task is impaired, indicative of a breakdown of PE. These behavioral results establish the ferret as a suitable animal model for subsequent investigation of the neural basis for the PE and, more generally, for listening in complex environments.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust through a Principal Research Fellowship (WT076508AIA) to A.J.K. We are grateful to Aideen Carroll, Sue Spires, Peter Keating, Dan Kumpik, Victoria Bajo, and Fernando Nodal for their contributions to behavioral testing.
REFERENCES
- 1. Backer, K. C. , Hill, K. T. , Shahin, A. J. , and Miller, L. M. (2010). “ Neural time course of echo suppression in humans,” J. Neurosci. 30, 1905–1913. 10.1523/JNEUROSCI.4391-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bianchi, F., Verhulst, S., and Dau, T. (2013). “ Experimental evidence for a cochlear source of the precedence effect,” J. Assoc. Res. Otolaryngol. 14, 767–779. 10.1007/s10162-013-0406-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bishop, C. W. , London, S., and Miller, L. M. (2011). “ Visual influences on echo suppression,” Curr. Biol. 21, 221–225. 10.1016/j.cub.2010.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blauert, J. (1997). Spatial Hearing the Psychophysics of Human Sound Localization (MIT Press, Cambridge, MA: ), pp. 1–494. [Google Scholar]
- 5. Blauert, J., and Braasch, J. (2005). “ Acoustic communication: The precedence effect,” in Proceedings of the Forum Acusticum 2005, Budapest. [Google Scholar]
- 6. Brown, A. D. , and Stecker, G. C. (2013). “ The precedence effect: Fusion and lateralization measures for headphone stimuli lateralized by interaural time and level differences,” J. Acoust. Soc. Am. 133, 2883–2898. 10.1121/1.4796113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifton, R. K. (1987). “ Breakdown of echo suppression in the precedence effect,” J. Acoust. Soc. Am. 82, 1834–1835. 10.1121/1.395802 [DOI] [PubMed] [Google Scholar]
- 8. Clifton, R. K. , and Freyman, R. L. (1989). “ Effect of click rate and delay on breakdown of the precedence effect,” Percept. Psychophys. 46, 139–145. 10.3758/BF03204973 [DOI] [PubMed] [Google Scholar]
- 9. Clifton, R. K. , Freyman, R. L. , Litovsky, R. Y. , and McCall, D. (1994). “ Listeners' expectations about echoes can raise or lower echo threshold,” J. Acoust. Soc. Am. 95, 1525–1533. 10.1121/1.408540 [DOI] [PubMed] [Google Scholar]
- 10. Damaschke, J., Riedel, H., and Kollmeier, B. (2005). “ Neural correlates of the precedence effect in auditory evoked potentials,” Hear. Res. 205, 157–171. 10.1016/j.heares.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 11. Dent, M. L. , and Dooling, R. J. (2003). “ Investigations of the precedence effect in budgerigars: Effects of stimulus type, intensity, duration, and location,” J. Acoust. Soc. Am. 113, 2146–2158. 10.1121/1.1558391 [DOI] [PubMed] [Google Scholar]
- 12. Dent, M. L. , and Dooling, R. J. (2004). “ The precedence effect in three species of birds (Melopsittacus undulatus, Serinus canaria, and Taeniopygia guttata),” J. Comp. Psychol. 118, 325–331. 10.1037/0735-7036.118.3.325 [DOI] [PubMed] [Google Scholar]
- 13. Dimitrijevic, A., and Stapells, D. R. (2006). “ Human electrophysiological examination of buildup of the precedence effect,” Neuroreport 17, 1133–1137. 10.1097/01.wnr.0000223386.44081.ec [DOI] [PubMed] [Google Scholar]
- 14. Djelani, T., and Blauert, J. (2000). “ Some new aspects of the buildup and breakdown of the precedence effect,” in Physiological and Psychophysical Bases of Auditory Function, edited by Breebaart D. J., Houtsma A. J. M., Kohlrausch A., Prijs V., and Schoonhoven R. (Shaker Publications, Maastricht, The Netherlands: ), pp. 200–207. [Google Scholar]
- 15. Djelani, T., and Blauert, J. (2001). “ Investigations into the build–up and breakdown of the precedence effect,” Acta Acust. Acust. 87, 253–261. [Google Scholar]
- 16. Fitzpatrick, D. C. , Kuwada, S., Kim, D. O. , Parham, K., and Batra, R. (1999). “ Responses of neurons to click-pairs as simulated echoes: Auditory nerve to auditory cortex,” J. Acoust. Soc. Am. 106, 3460–3472. 10.1121/1.428199 [DOI] [PubMed] [Google Scholar]
- 17. Freyman, R. L. , Clifton, R. K. , and Litovsky, R. Y. (1991). “ Dynamic processes in the precedence effect,” J. Acoust. Soc. Am. 90, 874–884. 10.1121/1.401955 [DOI] [PubMed] [Google Scholar]
- 18. Freyman, R. L. , and Keen, R. (2006). “ Constructing and disrupting listeners' models of auditory space,” J. Acoust. Soc. Am. 120, 3957–3965. 10.1121/1.2354020 [DOI] [PubMed] [Google Scholar]
- 19. Gardner, M. B. (1968). “ Historical background of the Haas and-or precedence effect,” J. Acoust. Soc. Am. 43, 1243–1248. 10.1121/1.1910974 [DOI] [PubMed] [Google Scholar]
- 20. Grantham, D. W. (1996). “ Left-right asymmetry in the buildup of echo suppression in normal-hearing adults,” J. Acoust. Soc. Am. 99, 1118–1123. 10.1121/1.414596 [DOI] [PubMed] [Google Scholar]
- 21. Haas, H. (1951). “ Über den Einfluss eines Einfachechos auf die Hörsamkeit von Sprache” (“On the influence of a single echo on the audibility of speech”), Acustica 1, 49–58. [Google Scholar]
- 22. Kalmykova, I. V. (1993). “ Investigation of the precedence effect in the cat auditory system,” Sensory Syst. 7, 208–211. [Google Scholar]
- 23. Keen, R., and Freyman, R. L. (2009). “ Release and re-buildup of listeners' models of auditory space,” J. Acoust. Soc. Am. 125, 3243–3252. 10.1121/1.3097472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keller, C. H. , and Takahashi, T. T. (1996). “ Responses to simulated echoes by neurons in the barn owl's auditory space map,” J. Comp. Physiol. A 178, 499–512. 10.1007/BF00190180 [DOI] [PubMed] [Google Scholar]
- 25. King, A. J. , Bajo, V. M. , Bizley, J. K. , Campbell, R. A. A. , Nodal, F. R. , Schulz, A. L. , and Schnupp, J. W. H. (2007). “ Physiological and behavioral studies of spatial coding in the auditory cortex,” Hear. Res. 229, 106–115. 10.1016/j.heares.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. King, A. J. , Dahmen, J. C. , Keating, P., Leach, N. D. , Nodal, F. R. , and Bajo, V. M. (2011). “ Neural circuits underlying adaptation and learning in the perception of auditory space,” Neurosci. Biobehav. Rev. 35, 2129–2139. 10.1016/j.neubiorev.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krumbholz, K., and Nobbe, A. (2002). “ Buildup and breakdown of echo suppression for stimuli presented over headphones—the effects of interaural time and level differences,” J. Acoust. Soc. Am. 112, 654–663. 10.1121/1.1490594 [DOI] [PubMed] [Google Scholar]
- 28. Litovsky, R. Y. , Colburn, H. S. , Yost, W. A. , and Guzman, S. J. (1999). “ The precedence effect,” J. Acoust. Soc. Am. 106, 1633–1654. 10.1121/1.427914 [DOI] [PubMed] [Google Scholar]
- 29. Litovsky, R. Y. , and Yin, T. C. (1998). “ Physiological studies of the precedence effect in the inferior colliculus of the cat. I. Correlates of psychophysics,” J. Neurophysiol. 80, 1285–1301. [DOI] [PubMed] [Google Scholar]
- 30. Matzke, D., and Wagenmakers, E.-J. (2009). “ Psychological interpretation of the ex-Gaussian and shifted Wald parameters: A diffusion model analysis,” Psychon. Bull Rev. 16, 798–817. 10.3758/PBR.16.5.798 [DOI] [PubMed] [Google Scholar]
- 31. Nodal, F. R. , Bajo, V. M. , Parsons, C. H. , Schnupp, J. W. , and King, A. J. (2008). “ Sound localization behavior in ferrets: Comparison of acoustic orientation and approach-to-target responses,” Neuroscience 154, 397–408. 10.1016/j.neuroscience.2007.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pecka, M., Zahn, T. P. , Saunier-Rebori, B., Siveke, I., Felmy, F., Wiegrebe, L., Klug, A., Pollak, G. D. , and Grothe, B. (2007). “ Inhibiting the inhibition: A neuronal network for sound localization in reverberant environments,” J. Neurosci. 27, 1782–1790. 10.1523/JNEUROSCI.5335-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perrott, D. R. , Strybel, T. Z. , and Manligas, C. L. (1987). “ Conditions under which the Haas precedence effect may or may not occur,” J. Aud. Res. 27, 59–72. [PubMed] [Google Scholar]
- 34. Rabinowitz, N. C. , Willmore, B. D. B. , King, A. J. , and Schnupp, J. W. H. (2013). “ Constructing noise-invariant representations of sound in the auditory pathway,” PLoS Biol. 11, e1001710. 10.1371/journal.pbio.1001710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ratcliff, R. (1978). “ A theory of memory retrieval,” Psychol. Rev. 85, 59–108. 10.1037/0033-295X.85.2.59 [DOI] [Google Scholar]
- 36. Ratcliff, R., and McKoon, G. (2008). “ The diffusion decision model: Theory and data for two-choice decision tasks,” Neural. Comput. 20, 873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanders, L. D. , Zobel, B. H. , Freyman, R. L. , and Keen, R. (2011). “ Manipulations of listeners' echo perception are reflected in event-related potentials,” J. Acoust. Soc. Am. 129, 301–309. 10.1121/1.3514518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spierer, L., Bourquin, N. M.-P. , Tardif, E., Murray, M. M. , and Clarke, S. (2009). “ Right hemispheric dominance for echo suppression,” Neuropsychologia 47, 465–472. 10.1016/j.neuropsychologia.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 39. Thurlow, W. R. , and Parks, T. E. (1961). “ Precedence-suppression effects for two click sources,” Percept. Mot. Skills 13, 7–12. 10.2466/pms.1961.13.1.7 [DOI] [Google Scholar]
- 40. Tollin, D. J. , McClaine, E. M. , and Yin, T. C. T. (2010). “ Short-latency, goal-directed movements of the pinnae to sounds that produce auditory spatial illusions,” J. Neurophysiol. 103, 446–457. 10.1152/jn.00793.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tollin, D. J. , and Yin, T. C. T. (2003). “ Psychophysical investigation of an auditory spatial illusion in cats: The precedence effect,” J. Neurophysiol. 90, 2149–2162. 10.1152/jn.00381.2003 [DOI] [PubMed] [Google Scholar]
- 42. Wallach, H., Newman, E. B. , and Rosenzweig, M. R. (1949). “ The precedence effect in sound localization,” Am. J. Psychol. 62, 315–336. 10.2307/1418275 [DOI] [PubMed] [Google Scholar]
- 43. Wolf, M., Schuchmann, M., and Wiegrebe, L. (2010). “ Localization dominance and the effect of frequency in the Mongolian gerbil, Meriones unguiculatus,” J. Comp. Physiol. A 196, 463–470. 10.1007/s00359-010-0531-7 [DOI] [PubMed] [Google Scholar]
- 44. Xia, J., Brughera, A., Colburn, H. S. , and Shinn-Cunningham, B. (2010). “ Physiological and psychophysical modeling of the precedence effect,” J. Assoc. Res. Otolaryngol. 11, 495–513. 10.1007/s10162-010-0212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang, X., and Grantham, D. W. (1997). “ Echo suppression and discrimination suppression aspects of the precedence effect,” Percept. Psychophys. 59, 1108–1117. 10.3758/BF03205525 [DOI] [PubMed] [Google Scholar]