Figure 7. Model of the action of HSF2 as a mediator of radial neuronal migration defects upon fetal alcohol exposure.
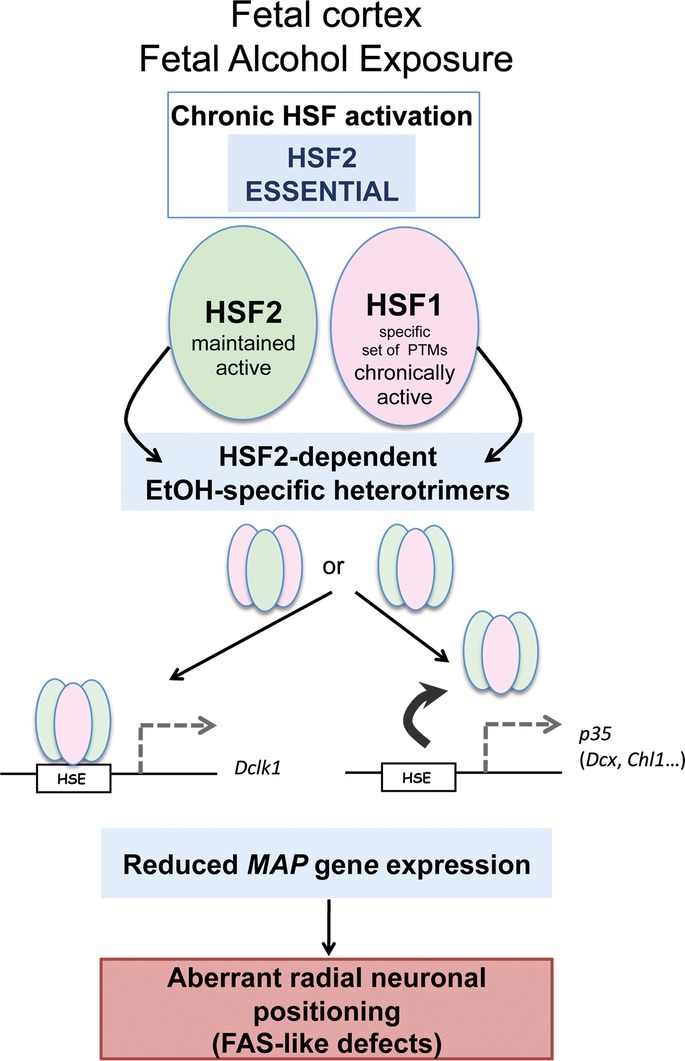
Fetal alcohol exposure leads to the maintenance of HSF2 activity and to persistent HSF1 activation, through an alcohol-specific set of HSF1 post-translational modifications (lack of acetylation and hyperphosphorylation, and limited sumoylation). HSF2 is essential for the activation of HSF1 in the developing brain cortex (but not in iMEFs, for example). This leads to the formation of alcohol-specific heterotrimers (HSF1 and HSF2 monomers in pink and green, respectively) that bind to HSF2 target genes involved in neuronal migration (which are bound by HSF2 homotrimers under normal conditions) and disturb their expression. Alternatively, the formation of heterotrimers prevents binding to the HSE (arrow). In the absence of HSF2, these disturbances are less pronounced than in wild-type cortices, and defects in the positioning of neurons are less severe. Thus, through its ability to steer HSF1 activation in the developing cortex, HSF2 is a mediator of the neuronal migration defect characteristics of fetal alcohol exposure in FAS models.
