Figure 8.
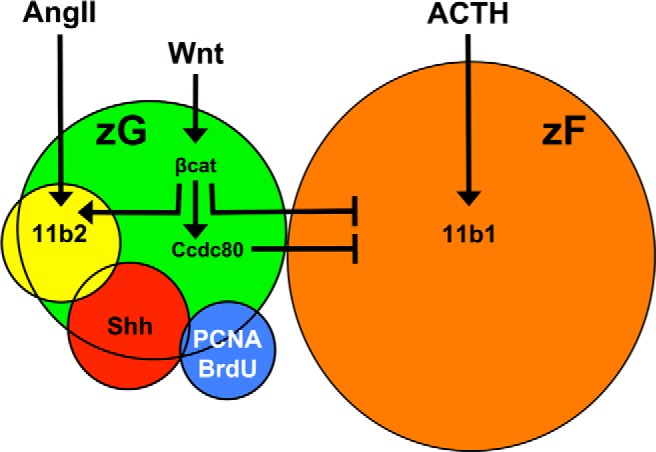
Integration of Wnt and endocrine signals specify zG vs zF gene expression. The Wnt-responsive population (green) is heterogeneous, consisting of differentiated Cyp11b2-expressing cells (yellow), Shh-producing progenitor cells (red), and few actively proliferating cells (blue) but no differentiated Cyp11b1-expressing cells of the zF (orange). Angiotensin II (AngII) and ACTH promote steroidogenesis in the zG and zF, respectively, defining the differentiated state of the two adrenocortical cell types. Wnt signals stimulate βcat, which inhibits the transcription of zF-associated steroidogenic genes and transcriptionally activates Ccdc80, a secreted protein that in turn also inhibits zF-associated steroidogenic gene expression. The rules that govern the balance the paracrine (Wnt/Ccdc80) and endocrine (ACTH/AngII) signals in this model are predicted to involve temporal and spatial cues. Coupled with the known role of βcat in the regulation of pro-zG genes, these data collectively support a role of Wnt signaling in facilitating the unidirectional centripetal differentiation of adrenocortical progenitor cells. Specifically, Wnt-mediated inhibition of zF-associated steroidogenesis, maintenance of the progenitor cell pool, and priming of zG cell fate assures the differentiation of adrenocortical progenitor cells into Cyp11b2-expressing zG cells before becoming Cyp11b1-expressing zF cells as supported by recent work. See Discussion for details.
