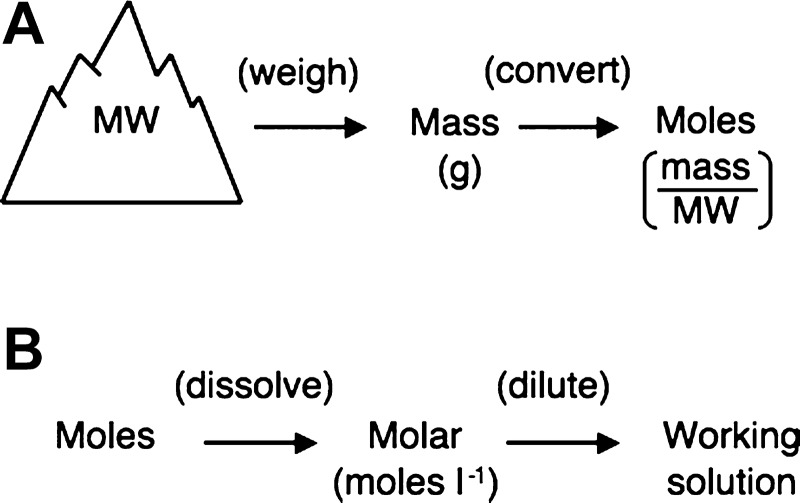
Fig. 1.
Relationships between key definitions and units. All compounds have a molecular weight (MW) determined by their chemical composition. A: the mass of the compound is converted to the equivalent number of moles by dividing the mass of the compound by its MW. B: the mass of compound dissolved in a solute is expressed as a molarity, i.e., the number of moles per liter of solution. Stock solutions can be diluted to yield working solutions.