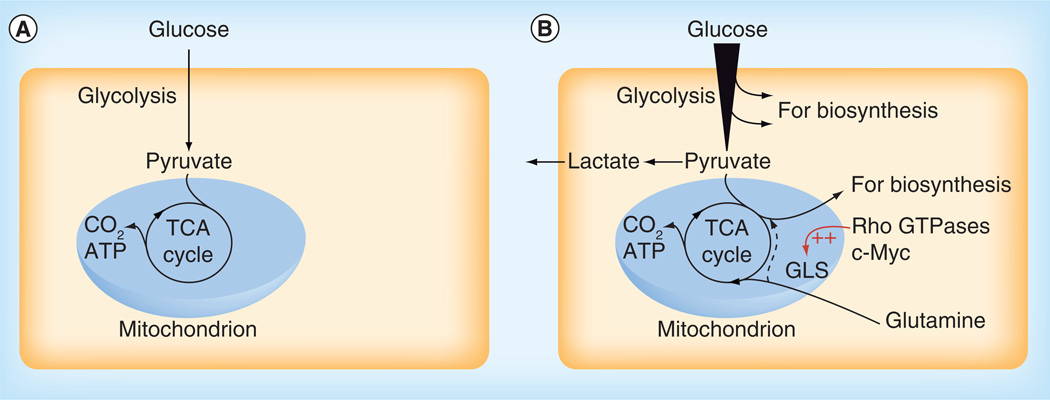
Figure 1. Cell proliferation requires metabolic reprogramming.
(A) In non-proliferating cells under aerobic conditions, metabolic fuels such as glucose typically undergo complete oxidation to CO2 in mitochondria via the TCA cycle. Energy released during this series of reactions is used to generate a proton electrochemical gradient across the inner mitochondrial membrane, which in turn drives ATP synthesis. (B) In proliferating cells there is an increased demand for precursors for protein, nucleotide and lipid production, in addition to ATP. Nutrient uptake is consequently enhanced and metabolic intermediates are diverted from glycolysis and the TCA cycle into biosynthetic pathways. For example, citrate from the TCA cycle can be exported from the mitochondrion to support lipogenesis in the cytosol. Reduction of pyruvate to lactate, catalyzed by lactate dehydrogenase, regenerates NAD+ to sustain glycolytic flux. Glutamine often serves as an anaplerotic substrate to maintain TCA cycle function, through its conversion by GLS and glutamate dehydrogenase to the TCA cycle intermediate α-ketoglutarate. Anaplerotic α-ketoglutarate can undergo oxidative metabolism in the TCA cycle or, during hypoxia or in cells with mitochondrial defects, reductive metabolism to citrate to support biosynthesis (dashed line).
TCA: Tricarboxylic acid.