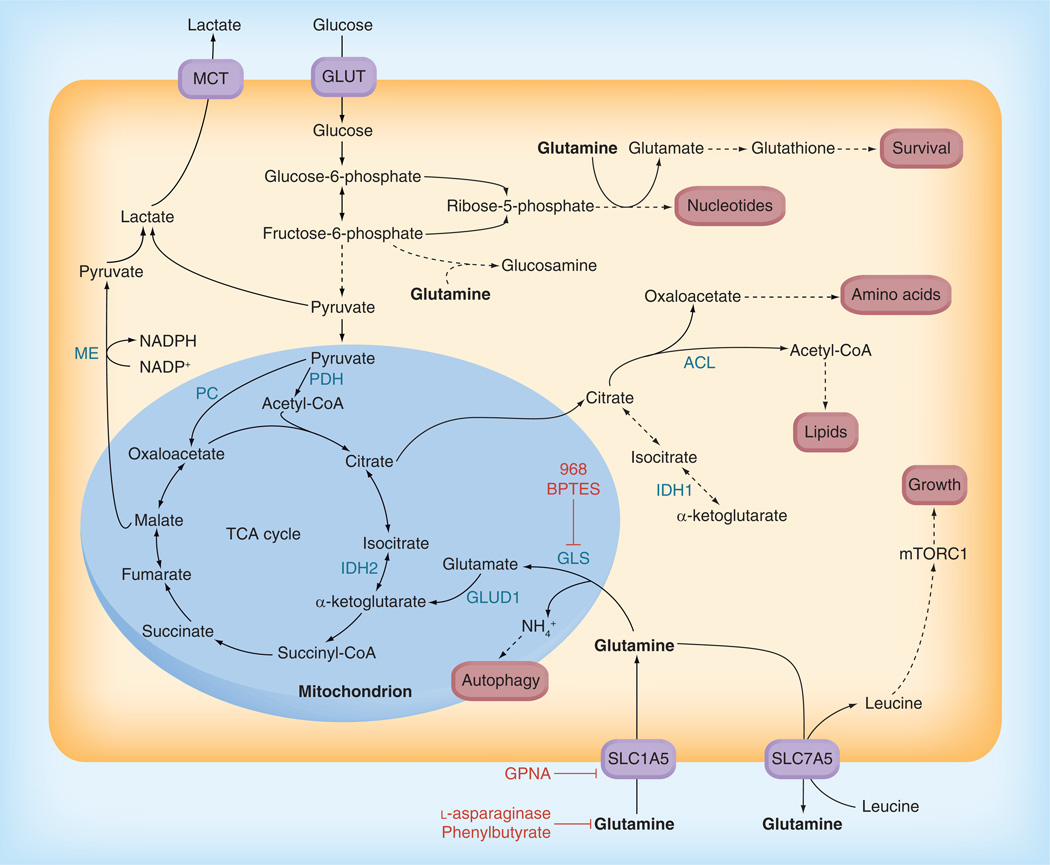
Figure 3. Core pathways of cellular glutamine metabolism.
Core pathways of glutamine metabolism, and their intersections with glucose metabolism, are depicted. 968 BPTES, GPNA, l-asparaginase and phenylbutyrate are inhibitors that target aspects of glutamine metabolism, and are shown in red. Glutamine is an essential nutrient for several processes that are important for cancer cell proliferation and survival. It serves as a nitrogen donor in nucleotide and glucosamine synthesis, a precursor for glutathione synthesis and a key anaplerotic substrate for maintenance of TCA cycle flux. Extracellular glutamine supplies can be depleted by l-asparaginase and by phenylbutyrate. Cellular uptake of glutamine is mediated by the transporter SLC1A5, which can be inhibited by the glutamine analog l-γ-glutamyl-p-nitroanilide. 968 and BPTES disrupt glutamine catabolism by allosterically inhibiting GLS.
ACL: ATP-citrate lyase; GLUD: Glutamate dehydrogenase; GLUT: Glucose transporter; IDH: Isocitrate dehydrogenase;
MCT: Monocarboxylate transporter; ME: Malic enzyme; PC: Pyruvate carboxylase; PDH: Pyruvate dehydrogenase; TCA: Tricarboxylic acid.