Figure 2.
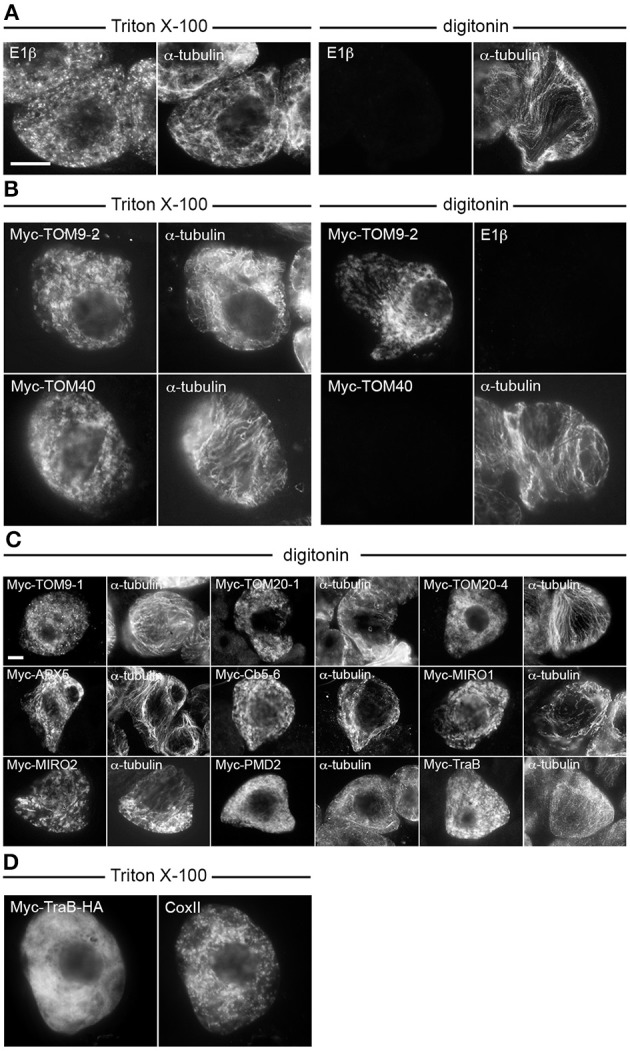
Topological mapping of selected A. thaliana OMM-TA proteins in differentially permeabilized BY-2 cells. Non-transformed (A) or transiently-transformed (B–D) BY-2 cells were formaldehyde fixed and permeabilized (as indicated above each set of images) with either Triton X-100, which perforates all cellular membranes, or, digitonin, which selectively permeabilizes the plasma membrane, then cells were processed for immuno-epifluorescence microscopy. Also indicated in each panel is the name of the immunostained transiently-expressed Myc-tagged protein or endogenous protein (i.e., E1β, CoxII or α-tubulin). Note that the presence or absence of immunofluorescence reflects whether the protein (epitope) was accessible to the applied antibodies. For instance, similar to α-tubulin in cytoplasmic microtubules (A), N-terminal Myc-tagged TOM9-2 (B) and all other known or putative TA proteins examined (C), but not endogenous E1β in the mitochondrial matrix or the control protein, Myc-TOM40 (B), were immunodetected in digitonin-permeabilized cells. Note also in (D) that Myc-TraB-HA did not colocalize with endogenous mitochondrial CoxII, indicating that the expressed protein, unlike Myc-TraB (Figure 1) is not properly targeted to mitochondria. Bar in (A,C) = 10 μm.
