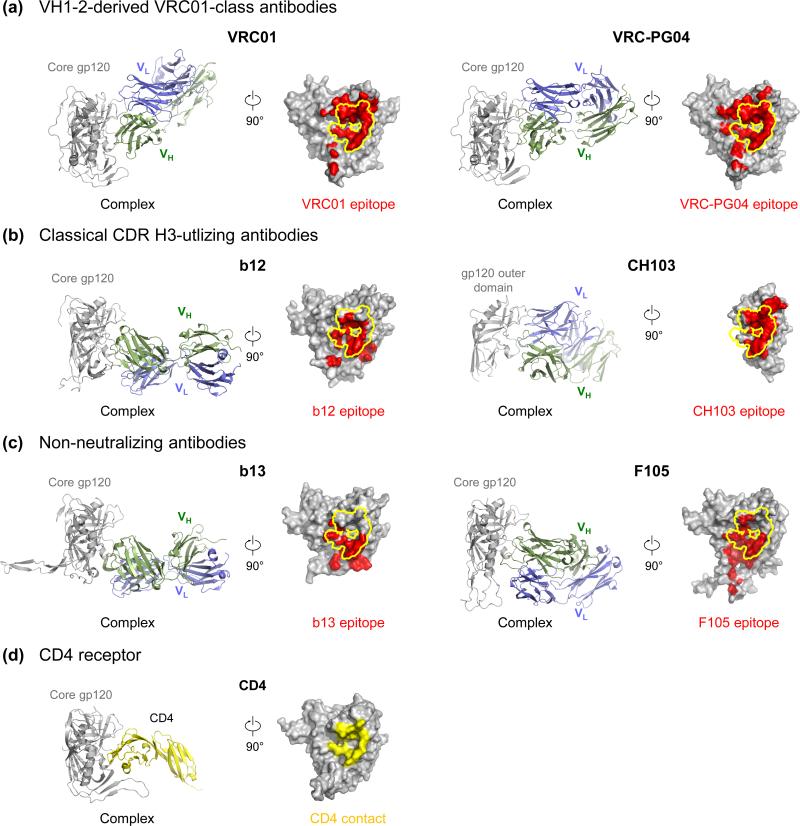
Figure 2. Antibody recognition at the CD4-binding site of HIV-1 gp120.
The structures of gp120 in complex with CD4-binding site antibodies (a-c) or CD4 (d) are shown in cartoon diagrams with HIV-1 gp120 colored in gray, antibody-heavy chain colored in green, antibody-light chain colored in blue, and CD4 colored in yellow. The epitopes of antibodies are highlighted in red on gp120 surface with the outer domain-contact site for CD4 outlined in yellow (a-c). The site of vulnerability defined as the outer domain contact of CD4 on HIV-1 gp120 is shown as a yellow surface (d). Effective neutralizers, either VH1-2-derived VRC01-class antibodies [6,31] or canonical CDR H3-utilizing antibodies [30,32], target this vulnerable site with high precision. In contrast, non-neutralizing CD4-binding site antibodies, such as b13 and F105 [33], only partially contact the site of vulnerability and induce significant conformational changes on monomeric gp120.