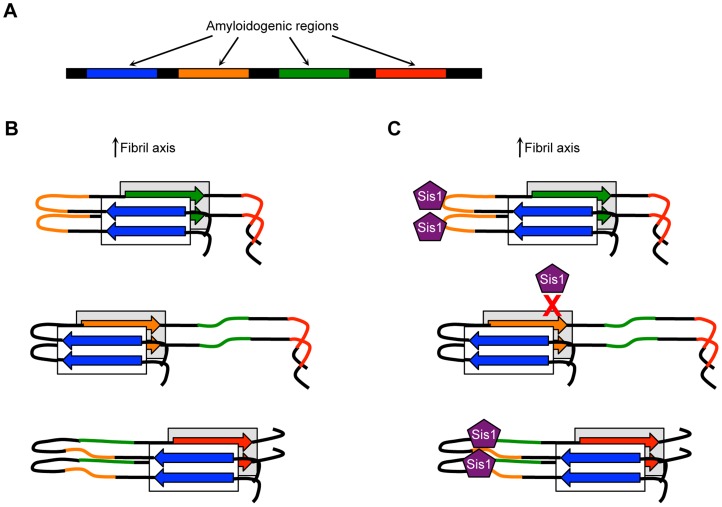
Figure 1. Model showing how distinct amyloidogenic regions could influence amyloid polymorphism and associated phenotypic variation.
(A) A single protein can have multiple amyloidogenic regions (colored as blue, orange, green, and red) that are not adjacent in the primary structure. (B) These regions can influence amyloid packing in a variety of ways, with nonadjacent regions possibly forming the amyloid core. (C) If a particular amyloidogenic region represents a chaperone-binding site (e.g., the Hsp40 Sis1 has affinity for the orange region), this region is exposed and available for binding in certain structures (top and bottom) but not others (middle). In addition, chaperone or cofactor binding prior to amyloid folding might influence the range of amyloid structures that can form or propagate, thereby providing a mechanism by which genetic and environmental modifiers might alter amyloid structure.