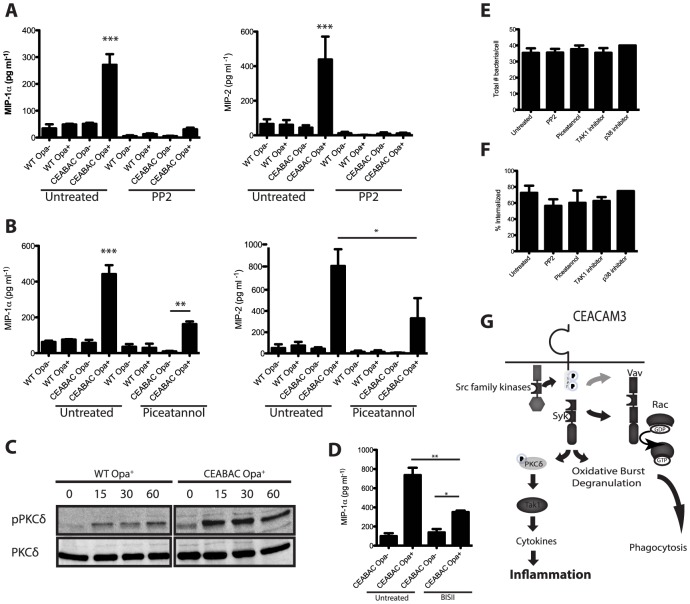
Figure 5. CEACAM3 signaling is required for the PMN cytokine response to N. gonorrhoeae.
Inhibition of Src-family kinases and Syk leads to decreased cytokine production by infected PMNs. (A, B) WT and CEABAC PMNs were left untreated or pre-incubated with (A) Src-family kinase inhibitor (PP2, 10 µM), or (B) Syk inhibitor (piceatannol, 50 µM). PMNs were then infected with either non-opaque (Opa−) or Opa-expressing (Opa+) N. gonorrhoeae, and MIP-1α and MIP-2 levels were measured 3 h post infection. N≥3, error bars represent SEM. One-Way ANOVA was performed for relevant samples, *P<0.05, **P<0.01, ***P<0.001 (C) Opa+ N. gonorrhoeae infection leads to phosphorylation of PKCδ. WT and CEABAC PMNs were infected with Opa+ N. gonorrhoeae (MOI = 10 bacteria/PMN) for times indicated times. PKCδ activation was measured by immunoblot using phospho-PKCδ antibody. Immunoblot for PKCδ indicates equal protein loading. (D) CEABAC PMNs were left untreated or pre-incubated with PKC inhibitor (BIS II, 10 µM). PMNs were then infected with Opa− or Opa+ N. gonorrhoeae, and MIP-1α levels were measured 3 h post infection. One-Way ANOVA (with Tukey's post-test) was performed for relevant samples, *P<0.05, **P<0.01, ***P<0.001. Unless otherwise indicated, stars indicate significance against all other conditions. (E, F) Inhibition of SFK, Syk, TAK1 or p38 does not affect bacterial binding (E) or phagocytosis (F). WT and CEABAC PMNs were left untreated or pre-incubated with indicated inhibitors. PMNs were then infected with Opa+ N. gonorrhoeae (MOI = 25) for 30 min. Intracellular and total bacteria were differentially stained, and quantified via immunofluorescence microscopy. (G) Schematic representation of proposed signaling pathway resulting in bacterial engulfment, activation of oxidative burst and degranulation, and cytokine production.