Abstract
Objective
Comparing the diagnostic value of multi-sequential cardiac magnetic resonance imaging (CMR) with endomyocardial biopsy (EMB) for sub-clinical cardiac allograft rejection.
Methods
One hundred and forty-six examinations in 73 patients (mean age 53 ± 12 years, 58 men) were performed using a 1.5 Tesla system and compared to EMB. Examinations included a STIR (short tau inversion recovery) sequence for calculation of edema ratio (ER), a T1-weighted spin-echo sequence for assessment of global relative enhancement (gRE), and inversion-recovery sequences to visualize late gadolinium enhancement (LGE). Histological grade ≥1B was considered relevant rejection.
Results
One hundred and twenty-seven (127/146 = 87 %) EMBs demonstrated no or mild signs of rejection (grades ≤1A) and 19/146 (13 %) a relevant rejection (grade ≥1B). Sensitivity, specificity, positive predictive, and negative predictive values were as follows: ER: 63 %, 78 %, 30 %, and 93 %; gRE: 63 %, 70 %, 24 %, and 93 %; LGE: 68 %, 36 %, 13 %, and 87 %; with the combination of ER and gRE with at least one out of two positive: 84 %, 57 %, 23 %, and 96 %. ROC analysis revealed an area under the curve of 0.724 for ER and 0.659 for gRE.
Conclusion
CMR parameters for myocarditis are useful to detect sub-clinical acute cellular rejection after heart transplantation. Comparable results to myocarditis can be achieved with a combination of parameters.
Key Points
• Magnetic resonance imaging is useful for the assessment of cardiac allograft rejection.
• CMR has a high negative predictive value for exclusion of allograft rejection.
• Diagnostic performance is not yet good enough to replace endomyocardial biopsy.
Keywords: Heart/transplantation, Graft rejection/diagnosis, Magnetic resonance imaging, Inflammation, Myocarditis
Introduction
Endomyocardial biopsy (EMB) is still considered to be the gold standard for the diagnosis of acute cellular rejection (ACR) after orthotopic heart transplantation (HTx), despite many studies also demonstrating promising results for cardiac magnetic resonance imaging (CMR). However, right ventricular EMB has a number of limitations: (1) it is an inconvenient invasive procedure with the risk of rare but serious adverse events like cardiac tamponade or death; (2) substantial exposure to radiation; (3) sampling error; (4) interobserver variability; (5) wide variability of protocols to use this invasive interventional tool; (6) repeated EMB may result in cardiac scarring or venous thrombosis [1, 2]. Therefore, EMB is very often performed by clinical suspicion of a rejection only, or at least the frequency of EMB was drastically reduced in many centres compared to initial protocols. This change of paradigm is problematic in itself, because clinical non-apparent (sub-clinical) ACR is not detected [3]. However, repeated sub-clinical ACRs are known to trigger the development of cardiac allograft vasculopathy (CAV) [4], a major long-term cause of graft failure after HTx [5].
A history of acute cellular rejection is an important risk factor for cardiac allograft vasculopathy (CAV) [6–8], which is the leading cause of death in heart transplant recipients at 5 years post-transplant, accounting for up to 30 % of deaths [5]. CAV develops in a majority of transplanted hearts at a variable rate, sometimes as early as 3 months after transplantation [9].
In the early post-transplant period, immune mediators like endothelial damage and inflammatory processes contribute to intimal thickening of the vascular intima of the cardiac allograft vasculature [10]. Non-immunological factors play a propagative role in the progression of CAV in the later post-transplant period [11]. In this context, it is important to identify patients with inflammatory processes of acute cellular rejection (ACR) in the cardiac allograft as early as possible. Although most of these inflammatory processes of ACR are sub-clinical, these patients need to be monitored more closely and, additionally, the immunosuppressive therapy needs to be optimized [12].
In the revision of the ISHLT working formulation for histological grading of EMB, the classification has been simplified by combining several formerly distinct grades [3]. Grades 1A, 1B, and 2 have been combined into grade 2R. Yet, many transplant centres are still using the original classification to distinguish the lower histological grades of ACR in more detail compared to the revised version. This is supported by a study by Hollweg et al. [13], showing that grades 1A and 1B are indeed distinct entities with regard to gene expression. In particular, HTx patients with histological grade 1B showed an upregulated immune response. The authors concluded that there are overlapping pathological pathways between grade 1B and grade 3A or 3B of ACR.
On this account, it is an ongoing challenge to find an accurate and less invasive alternative to EMB for the diagnosis of ACR. Multi-sequential CMR has proven its diagnostic value to detect acute and chronic myocarditis by assessing myocardial edema, hyperemia, and necrosis [14, 15], which are also histological findings in ACR [16]. Therefore, CMR could be useful in this context as well. A couple of previous studies have compared single CMR parameters with EMB for the diagnosis of ACR after HTx [17]. Only one study used a multi-sequential CMR approach like in myocarditis in patients with moderate to severe ACR and demonstrated a high sensitivity and a moderate specificity [18].
In the present study, we wanted to evaluate the possibility to detect sub-clinical ACR – which is clinically relevant to decide if to start medical treatment or not – by a multi-sequential CMR protocol in comparison to EMB as the standard of reference.
Materials and methods
Patient selection
All patients from our tertiary care centre who were scheduled for a control EMB after heart transplantation from December 2008 until January 2012 were assessed for their study eligibility. Exclusion criteria included all contraindications for a contrast-enhanced CMR examination: Presence of cardiac pacemaker or implantable cardiac defibrillator, other MR incompatible devices or implants, claustrophobia, and a history of severe adverse reactions to gadolinium-based contrast agents.
Approval was granted by the ethics committee of the University of Leipzig. Written informed consent was obtained from all patients. Patient characteristics are summarized in Table 1.
Table 1.
HTx patient characteristics. Abbreviations: ISHLT = International Society of Heart and Lung Transplantation, LV-EF = left-ventricular ejection fraction, LV-EDV = left-ventricular end-diastolic volume, LV-ESV = left-ventricular systolic volume
| All examinations (n = 146) | Histological Grade0 + 1A (ISHLT 1990) (n = 127) | Histological Grade ≥ 1B (ISHLT 1990) (n = 19) | Differences (p-value) | |
|---|---|---|---|---|
| Age (years) | 52 ± 12 | 52 ± 12 | 48 ± 14 | 0.12 |
| Sex (male/female) | 115/31 | 101/26 | 14/5 | 0.55 |
| Months post-transplant | 77 ± 21 | 69 ± 21 | 77 ± 21 | 0.13 |
| LV-EF (%) | 58 ± 9 | 58 ± 9 | 58 ± 10 | 0.87 |
| LV-EDV (ml) | 130 ± 34 | 131 ± 34 | 124 ± 28 | 0.57 |
| LV-ESV (ml) | 56 ± 24 | 56 ± 24 | 54 ± 22 | 0.74 |
Endomyocardial biopsies
Right ventricular EMBs were routinely performed six times in the first year post-transplantation period (day 14 and 1, 3, 6, 9, and 12 months post HTx) and once per year in the following years. The classification of the International Society for Heart and Lung Transplantation (ISHLT) working formulations 1990 was used for histological grading of ACR [3].
Immunosuppressive therapy
All patients received an induction therapy preoperatively with tacrolimus (1 mg p.o.), mycophenolate mofetil (1,000 mg p.o.) and corticosteroids (250 mg i.v.). Postoperatively immunosuppressive therapy was continued with tacrolimus (dosing to target blood concentration), mycophenolate mofetil (2 to 3 g/d) and corticosteroids (tapering regimen to 2.5 or 5 mg/d) until side effects occurred. Treatment of biopsy proven acute rejection in our centre was as follows: Histological grade 1A was not treated. Grades 1B or higher were treated with a daily steroid pulse of 500 mg methylprednisone for 3 days, followed by oral steroid doses of 100 mg daily, tapering down every 3 days by half of the dose until the standard steroid maintenance therapy was reached (5 mg/day). Anti-thymocyte globulin (1 mg/kg/body weight for 1 to 3 days) was administered in cases of steroid-resistant ACR or in cases of any cellular rejection with hemodynamic compromise.
Cardiac magnetic resonance imaging protocol
All 73 patients underwent CMR using a clinical 1.5 T MR (Philips Achieva, Philips medical systems, Best, the Netherlands) and a dedicated cardiac 32-channel phased-array surface coil. The T2-weighted images for the calculation of the edema ratio (ER) and the T1-weighted spin-echo images to calculate the global relative enhancement (gRE) were acquired using the body coil to avoid influence of signal heterogeneities on signal intensity measurements as previously described [14, 15]. This does, however, lead to a decreased signal-to-noise-ratio (SNR) in comparison to the cardiac surface coil.
For functional analysis, a steady-state free precession sequence was performed in the four- and two-chamber view and in a stack of short-axis slices covering the whole left ventricle. Acquisition was done during breath hold with retrospective ECG gating with the following parameters: TR 3.4 ms, TE 1.5 ms, flip angle 60°, slice thickness 8 mm, field of view 320–400 mm, matrix 256x320.
ECG-gated T2-weighted short tau inversion recovery (STIR) images for ER calculations were acquired in short axis slices covering the whole left ventricle during end-diastole while free-breathing using a navigator technique and the same slice orientation as for the short-axis SSFP-slices with the following parameters: TR 2 R-R-intervals, TE 80 ms, flip angle 90°, slice thickness 8 mm, field of view 370x335 mm, matrix 512x512.
T1-weighted turbo spin-echo imaging was performed while free breathing starting 15 s after intravenous administration of 0.1 mmol/kg body weight of gadobutrol (Gadovist, Bayer HealthCare Pharmaceuticals, Berlin, Germany). Five transverse slices covering the left ventricle before and after contrast administration were acquired with the following parameters as previously described [6, 7]: TR 1 R-R interval, TE 15 ms, flip angle 90°, slice thickness 8 mm, field of view 300–350 mm, matrix 480x480.
Sequences for the analysis of LGE were acquired at least 10 min after the i.v. administration of contrast agent. A two-dimensional look-locker sequence was used to determine the optimal inversion time. Then the left ventricle was covered in four- and two-chamber orientation with retrospectively ECG-gated three-dimensional inversion recovery gradient echo sequence, TE 6.5 ms, TR 3.1 ms, flip angle 15°, reconstructed voxel size 1.29 mm, field of view 370 mm, matrix 280x280.
Furthermore, a phase-sensitive inversion recovery (PSIR) sequence was acquired in short-axis slices covering the whole ventricle with TE 3.6 ms, TR 1.2 ms, flip angle 15°, FOV 480x382, reconstructed voxel size 1.8 mm, matrix 256x256, and retrospective ECG gating.
Assessment of left-ventricular size and function
Analysis of left ventricular function was performed in the short axis cine sequence with commercially available software (ViewForum R6.3V1L7 or Extended MR Workspace 2.6.3.4, Philips medical systems, Best, the Netherlands). End-diastolic and end-systolic endocardial contours were drawn manually by an experienced radiologist, with papillary muscles included in the blood pool. Using the Simpson method, left-ventricular end-diastolic volume (LV-EDV) and end-systolic volume (LV-ESV), as well as left-ventricular ejection fraction (LV-EF), were calculated.
CMR assessment of inflammation
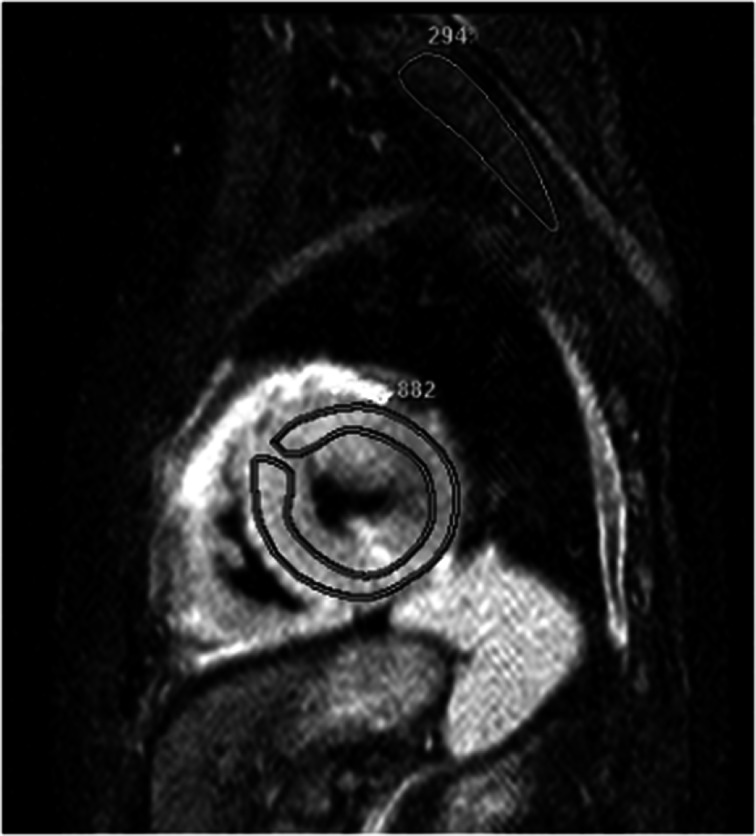
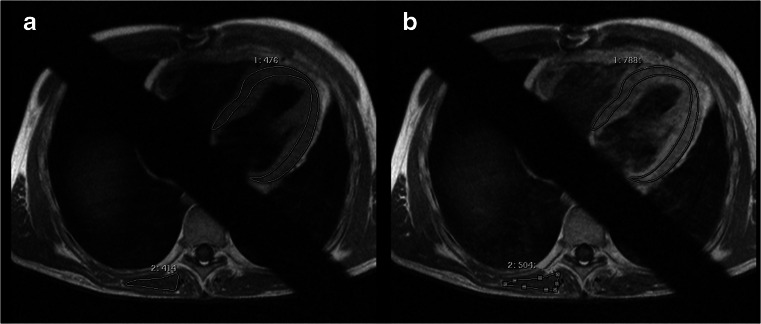
The ER and the gRE were calculated as previously described [14, 15] from the STIR and T1-weighted TSE sequences. Using commercially available software (ViewForum R6.3V1L7 or Extended MR Workspace 2.6.3.4, Philips medical systems, Best, the Netherlands) two spline-based regions of interest were drawn on the same slice encompassing the left-ventricular myocardium and the latissimus dorsi or erector spinae muscle, respectively. This was repeated on three slices in the basal, midventricular and apical myocardium for calculation of the ER (Fig. 1). In the same way, regions of interest were drawn on one slice of the pre-contrast T1-weighted sequence and copied to the corresponding image acquired after adminstration of contrast agent (Fig. 2). ER was then defined as the mean signal intensity of the myocardium (SImyo) divided by the mean signal intensity of skeletal muscle (SIskm): ER = SImyo / SIskm. For statistical analysis, the average ER of three slices was obtained. From the signal intensities obtained from the T1 sequence, gRE of myocardium compared to skeletal muscle was calculated as defined in previous studies [14, 15]. Similar to the ER, the average of gRE was calculated from three different slices.
Fig. 1.
Short-axis STIR image with ROIs drawn in the myocardium and skeletal muscle. Edema Ratio (ER) is 3.0
Fig. 2.
Transverse T1-weighted TSE sequence before (a) and after contrast injection (b) with ROIs drawn in the myocardium and skeletal muscle. Global relative enhancement (gRE) is 3.0
Statistics
All results were given as mean and standard deviations (SD). Q-Q plots and Kolomogorov-Smirnov test were used to analyse distribution of data. Depending on whether the distribution was different from normality, the significance of differences was analysed using Student’s unpaired t-test, Wilcoxon test or Mann–Whitney rank sum test, where appropriate. Multiple groups were compared using the Kruskal–Wallis one-way analysis of variance for all but the patients with a histological grade 2 and 3A, as the numbers in these subgroups were too small (one and two patients, respectively). As LGE was only recorded as a binary variable (positive or negative), Fisher's exact test was used for comparison. Two-tailed P-values <0.05 were considered statistically significant. For evaluation in a clinical context, patients with a therapeutically relevant rejection (histological grade ≥1B) were compared against a control group including all patients with grades 0 or 1A. Regarding these two subgroups, sensitivity, specificity, NPV, and PPV were calculated using ER, gRE, LGE, and the cutoff values derived from receiver operating characteristic (ROC) analysis for the ER and gRE values (Fig. 3).
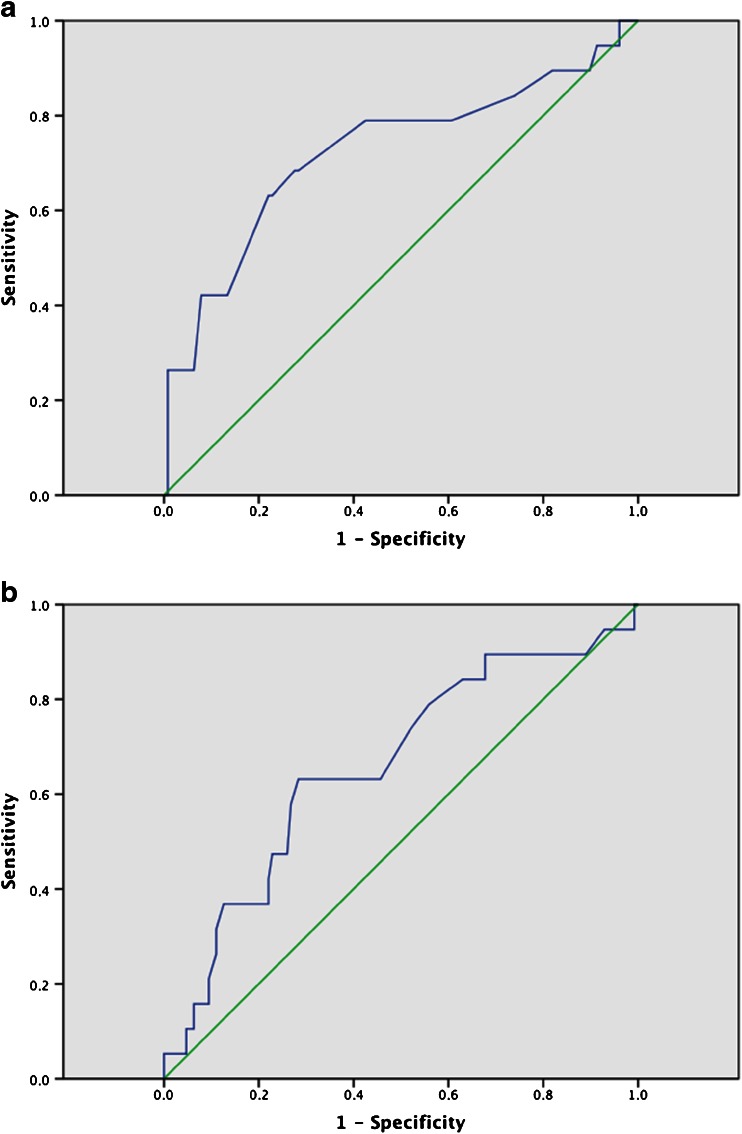
Fig. 3.
Receiver operator characteristic (ROC) analysis for the CMR parameters ER (a) and gRE (b) for the diagnosis of therapeutically relevant rejection (grade ≥ 1B) as compared to the results of EMB demonstrate good results for both parameters with an area under the curve (AUC) of 0.724 for the ER (a) and 0.659 for the gRE (b)
For the assessment of inter- and intra-observer variability, ER and gRE were calculated in 20 randomly selected examinations two times by the same radiologist and independently by a second radiologist. Analysis was made as described by Bland and Altman [19]. The coefficient of variation was calculated as the standard deviation of the mean difference between measurements, divided by the mean value for the specific parameter. All statistics were calculated using commercially available software (IBM SPSS, Version 19, IBM corporation, New York, NY, USA).
Results
Patient characteristics
In the period denoted above, 73 patients with cardiac allograft were scheduled for EMB and CMR on the same day. As some patients underwent CMR and EMB more than once during this period, a total of 168 examinations could be acquired. Twenty-two (22/168) CMR examinations were excluded from the study, because (1) the EMB material was not sufficient for histological grading according to the ISHLT guidelines (n = 18/168 [11 %]), or (2) CMR examinations were aborted too early due to patient discomfort (n = 4/168 [2.4 %]). In total, 146 examinations could be included in the study to compare CMR with EMB findings (Table 1).
Endomyocardial biopsy (EMB)
One hundred and twenty-seven (127/146 = 87 %) EMBs demonstrated no or mild signs of rejection, histological grade 0 (n = 50) and grade 1A (n = 77). In 19 examinations (19/146 = 13 %) of HTx patients, EMBs revealed relevant ACR (grade ≥1B) requiring medical anti-inflammatory treatment according to the ISHLT classification from 1990 [3]. Among these, there was one patient with histological grade 2 and two patients with grade 3A rejection. The other 16 samples were classified as histological grade 1B.
Cardiac magnetic resonance imaging
Functional and volumetric left-ventricular parameters
The mean LV-EF of all CMR examinations was within the normal range with 58 ± 9 %, as well as the mean LV-EDV and LV-ESV with 130 ± 56 ml and 56 ± 24 ml, respectively. There were no statistically significant differences between the different subgroups, patients with or without clinically relevant ACR (Table 1). Within the different histological subgroups, patients with histological grades 0 and 1A had a mean LV-EF of 58 ± 9 %, a mean LV-EDV of 131 ± 34 ml and LV-ESV of 56 ± 24 ml. Mean values for patients with histological grade ≥1B were 58 ± 10 %, 124 ± 28 ml and 54 ± 22 ml.
Receiver operator characteristic (ROC) analysis
The receiver operator characteristic analysis revealed an area under the curve (AUC) of 0.724 for ER and 0.659 for gRE (Fig. 3). Detailed analysis confirmed thresholds of 2.0 for ER and 4.5 for gRE as the most reasonable compromise between high specificity and sensitivity.
Edema ratio (ER)
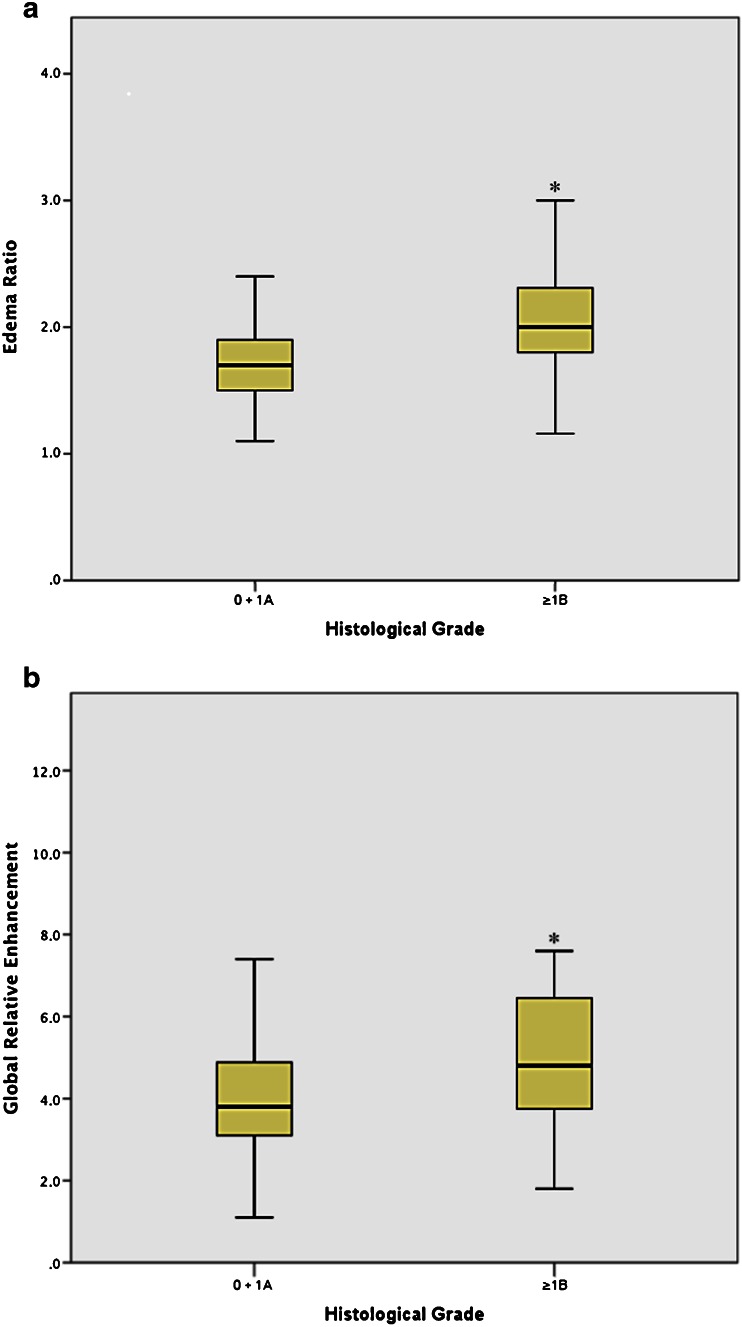
Mean and standard deviation of ER indicating edema were 1.8 ± 0.4 for all examinations and 1.7 ± 0.4 for histological grades 0-1A. Values for patients with a clinically relevant histological grade ≥1B were only slightly, but significantly (p < 0.05) elevated with a mean value of 2.1 ± 0.5 (Fig. 4a).
Fig. 4.
The box-plots of the ER (a) and gRE (b) in patients with histological grade 0 and 1A (n = 127) vs. patients with grade 1B or higher (n = 19) demonstrated with statistically significant differences (*p < 0.05)
For the Kruskal–Wallis test, a significant difference among the individual histological grades was observed (p = 0.03). Post hoc analysis showed significant differences between the histological grades 0 and 1B (p = 0.002), as well as between histological grades 1A and 1B, but not between grades 0 and 1A (p = 0.38). Regarding the two clinically distinct subgroups, ER in patients with therapeutically relevant rejection was significantly higher compared to controls (p = 0.002) (Fig. 4a). Using the standardized cutoff values for myocarditis [6, 7], the comparison of ER with the histological grade of rejection as the standard of reference had a sensitivity, specificity, PPV, and NPV of 63 %, 78 %, 30 %, and 93 % (Table 2).
Table 2.
Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) of different single and combined CMR parameters for diagnosis of therapeutically relevant acute rejection (grade ≥1B) as proven by EMB. Abbreviations: ER = edema ratio, gRE = global relative enhancement, LGE = late gadolinium enhancement
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| ER | 63 | 78 | 30 | 93 |
| gRE | 63 | 70 | 24 | 93 |
| LGE | 68 | 31 | 13 | 87 |
| 1 out of 3 (ER, gRE, LGE) | 95 | 18 | 15 | 96 |
| 2 out of 3 (ER, gRE, LGE) | 68 | 67 | 24 | 93 |
| 1 out of 2 (ER or gRE) | 84 | 57 | 23 | 96 |
Global relative myocardial enhancement (gRE)
Mean and standard deviation of gRE were 4.4 ± 1.8 for all examinations, 4.2 ± 1.7 for histological grades 0/1A, and 5.3 ± 2.3 (Fig. 4b) for patients with clinically relevant grade ≥1B, significantly higher than the published threshold of 4.0 [6, 7] for myocarditis as well as the higher cutoff (4.5) derived from our ROC analysis.
No significant differences between individual histological grades were found. However, gRE of patients with clinically relevant rejection (≥1B) differed significantly from patients without clinically relevant ACR (≤1A) with p = 0.025 (Fig. 4b). For the detection of histological grade ≥1B sensitivity, specificity, PPV, and NPV were 63 %, 70 %, 24 %, and 93 %, respectively (Table 2).
Late gadolinium enhancement (LGE)
LGE of the myocardium could be demonstrated in 101 of 146 MR examinations (69 %). Of these examinations, only 13 (9 %) coincided with a positive (grade ≥1B) result of the EMB. There was no significant difference in the occurrence of LGE between patients with or without therapeutically relevant rejection. Regarding the diagnosis of histological grade ≥1B, a moderate sensitivity of 68 % and NPV of 87 % was found, whereas specificity and PPV were low with 31 % and 13 %, respectively (Table 2).
Combination of CMR parameters
Best results regarding sensitivity and NPV were achieved by combining several of these parameters (Table 2). Defining an examination with at least one parameter out of three as positive, a very high sensitivity of 95 % and a very high NPV of 96 % could be achieved, though specificity was very low with only 18 %.
Defining either elevated ER and/or gRE as positive (at least one out of two) resulted in a high sensitivity of 84 %, moderate specificity of 57 % and an equally good NPV of 96 %.
Both patients with histological grade 3A rejection showed an elevated ER, gRE as well as LGE. CMR of the patient with grade 2 rejection was only noticeable for LGE whereas ER and gRE were within the normal range.
Inter- and intra-observer variability
For the intra-observer variability, mean difference ± standard deviation was 0.09 ± 0.23 for ER and −0.25 ± 1.13 for gRE, with the coefficient of variation being 12 % and 27 %, respectively.
In the inter-observer comparison, mean difference ± standard deviation was 0.03 ± 0.25 for ER and −0.85 ± 2.85 for gRE, with coefficients of variation of 14 % and 38 %.
Analysis of the Bland – Altman plots revealed an increase in intra- and interobserver differences in patients with higher gRE values.
Discussion
In the current study, a multi-sequential CMR protocol was used to assess cardiac allograft rejection. For therapeutically relevant rejection of ISHLT grade 1B or greater, a combination of cardiac ER and gRE achieved a good NPV 96 %. However, CMR parameters provided only a low PPV of 30 % at maximum due to a high number of false positives.
Endomyocardial biopsy (EMB) is still considered the gold standard for the detection and classification of ACR [3]. However, several CMR studies for the evaluation of ACR have been carried out since the late 1980s (Table 3) demonstrating promising results.
Table 3.
Previous studies on CMR in detection of ACR in correlation with histological grade with an overview of included CMR parameters. Abbreviations: absolute = calculation of absolute T1- or T2-relaxation time, SI = measurement of signal intensity, relative = calculation of relative signal intensity, visual = visual assessment of myocardial hyperintensity or enhancement, ER = edema ratio, gRE = global relative enhancement, NA = not applicable; 1 cellular infiltrate and myocytolysis, 2 difference of pre- and postcontrast signal intensity divided by precontrast signal intensity 3>=1 focus of myocyte necrosis, 4 ratio of myocardial and skeletal muscle signal intensity, 5 difference of signal intensities pre- and postcontrast , 6 difference of pre- and postcontrast relative signal intensity divided by postcontrast relative signal intensity, 7 signal intensity ≥ standard deviations above that of remote myocardium
| Study | Number of patients (n)/CMR examinations (n) | Functional Parameters (Y/N) | T2-w imaging | Sens/Spec | Histological Criteria | T1-w imaging no CM | Sens/Spec | Gd-enhancement | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| early | Sens/Spec | late | Sens/Spec | ||||||||
| Wisenberg 1987 [11] | 25/62 | Y | absolute | 93/96 | defined1 | absolute | 93/96 | - | - | - | - |
| Lund 1988 [12] | 9/35 | N | absolute | NA | unknown | - | - | - | - | - | - |
| Revel 1989 [16] | 29/33 | Y | SI | NA | Billingham [20] | - | - | - | - | - | - |
| Doornbos 1990 [18] | 14/42 | N | absolute | NA | Billingham [20] | - | - | - | - | - | - |
| Mousseaux 1993 [17] | 39/39 | N | absolute | NA | ISHLT 1990 [3] | - | - | relative2 | - | ||
| Smart 1993 [13] | 8/33 | N | relative | 100 | McAlister [21] | - | - | - | - | - | - |
| Marie 1998 [14] | 52/52 | N | absolute | 89/91 | ISHLT1990 [3] ≥2 | - | - | - | - | - | - |
| Marie 2001 [15] | 68/123 | N | absolute | 89/70 | ISHLT 1990 [3] ≥2 | - | - | - | - | - | - |
| Almenar 2003 [22] | 40/64 | Y | visual | NA | defined3 | relative4 | Absolute5 and relative6 | - | |||
| Taylor 2010 [10] | 50/66 | Y | ER | 55/90 | ISHLT 2004 [3] ≥2R | - | - | gRE | 82/79 | relative7 | - |
| Usman 2012 [23] | 53/68 | Y | absolute | 86.5/94.6 | ISHLT 2004 [3] ≥2R | - | - | - | - | - | - |
| Own results: 2013 | 73/146 | Y | ER | 63/78 | ISHLT ≥1B | - | NA | gRE | 63/70 | visual | 68/36 |
As early as 1987, a study of Wisenberg et al. [22] explored the potential of CMR in a small group of 25 patients after HTx. They found significantly increased T1- and T2-values, and an increased wall thickness in HTx patients compared to healthy controls. Using a threshold of two standard deviations above the mean, cases of rejection could be identified with high accuracy. From the numbers stated in their publication, a very high sensitivity of 93 % and specificity of 96 % could be calculated. However, a sophisticated histological grading system had not yet been established, so rejection was simply defined by the presence of cellular infiltrates and myocytolysis. This and the less efficient immunosuppressive therapy at that time – which resulted in a high prevalence (>50 %) of ACR with 13 out of 25 patients – make it difficult to compare these results with more recent CMR studies, which demonstrated less promising results.
Since then, several attempts have been made to increase the use of CMR in HTx patients, with T2-imaging being the most widely used technique [17]. Several studies could confirm a significant increase in absolute T2-values [23–25] or relative T2-signal [20] intensity in patients with ACR. But initial studies by Lund and Smart et al. were limited by the very small number of patients [20, 24] and undefined histological grading [24].
Two studies by Marie et al. included more patients and a histological grading according to the ISHLT recommendations [23, 25], just as the study presented here. While identical imaging parameters were used, the more recent study included a larger number of examinations (128 vs. 52). Both studies found the same sensitivity of 89 % for the detection of ACR (defined as a histological grade ≥2) with a specificity of 91 % [23] and 70 % [25], respectively. From our data, a lower sensitivity of 67 % and similar specificity of 73 % for the detection of histological grade ≥2 could be calculated. However, we could include only three patients of this subgroup in our study.
Studies by Revel [21], Mousseaux [26], and Doornbos et al. [27] revealed contradictory results demonstrating no association between T2-imaging and ACR. Among these, one study reported myocardial signal intensity measurements only [21] instead of absolute T2-relaxation time calculations or relative signal intensity measurements [20]. The studies by Mousseaux et al. [26] and Doornbos et al. [27] featured systolic image acquisition, which may degrade image quality [28].
Myocardial enhancement after contrast injection has been studied less often in the context of ACR. Studies by Mousseaux et al. [26] and Almenar et al. [29] were able to show a significant increase of myocardial contrast enhancement in patients with ACR. Comparison with our results is difficult as both studies used different histological and CMR criteria. Mousseaux et al. [26] defined myocardial enhancement as the difference between maximum signal intensity post- and pre-contrast, divided by signal intensity pre-contrast. Almenar et al. [29] defined relative signal intensity as the ratio of myocardial signal intensity to skeletal muscle intensity. Relative myocardial uptake was then calculated as the difference of post- and pre-contrast relative signal intensity, divided by post-contrast relative signal intensity. For patients with ≥1 focus of myocyte necrosis, a significantly higher relative myocardial uptake was found. Neither study provided cutoff values or diagnostic sensitivity or specificity for the diagnosis of ACR.
One possible approach to expand further the usefulness of CMR was the combination of several different imaging techniques in one examination, which was first introduced by Wisenberg et al. [22] in 1987 and was then almost abandoned for over more than ten years until Almenar´s [29] and Taylor´s [18] studies.
In our study, using a multi-sequential CMR protocol originally established for the detection of myocarditis [14, 15], a combination of ER and gRE proved to be valuable to rule out therapeutically relevant ACR with a sensitivity of 84 % and NPV of 96 %. Also, significant differences of both parameters were found between patients with a histological grade of rejection ≥1B compared to the histological grades of rejection 0 and 1A.
Echocardiographic measurements have shown that ventricular function is compromised in severe cases of rejection [30]. Yet, in our study, parameters of left-ventricular morphology and systolic function did not show a significant correlation with histological grades. This observation confirms results from previous studies [18, 29, 31] in HTx patients, but also on CMR of myocarditis patients [14, 15]. Neither Almenar and Taylor et al. nor Usman et al. found significant differences in ventricular function or volumes between patients with and without ACR. Thus, it seems that functional parameters are not sensitive enough to detect either sub-clinical ACR or myocarditis.
Left ventricular ER and gRE have been successfully implemented as parameters for the evaluation of myocardial inflammation previously [14, 15]. Our findings of moderate to good sensitivity and specificity of both CMR parameters are inferior to values published for acute myocarditis by Abdel-Aty et al. [32], but better than the results of Gutberlet et al. [15] for chronic myocarditis.
Thus, though the pathophysiological mechanisms underlying allograft rejection are different from those in acute myocarditis, it seems that the common findings of myocardial edema and hyperaemia lead to similar changes in CMR parameters.
It must be noted that most studies on CMR in myocarditis used a gRE cutoff value of 4.0 [14, 15, 32], while Taylor et al. found a cutoff value of 3.5 for the detection of ACR. Both are different from the optimal value of 4.5, which was found in our study via ROC analysis (Fig. 3). Because of the dependence on technical parameters such as field strength and vendor [33], it may be that this optimum cutoff value found in our study does not apply to different CMR systems and sequences.
A combination of the two parameters ER and gRE delivered the best diagnostic performance with an NPV of 96 %. To date, only one other study [18] has reported on the adoption of a similar multi-sequential CMR protocol for the evaluation of ACR.
In a smaller patient population (n = 50), using the same combined criteria approach of either elevated ER or gRE, a sensitivity of 100 % and specificity of 73 % were achieved for ACR and the revised ISHLT grading of rejection for grades 2R and higher.
In our patient population, sensitivity, specificity, PPV and NPV for histological grades 2 and higher were 67 %, 73 %, 5 %, 99 % for ER; 67 %, 66 %, 4 %, 99 % for gRE; and 100 %, 31 %, 3 %, 100 % for LGE. This was due to one patient with histological grade 2 showing normal values for ER and gRE. Yet, as only three patients with histological grade ≥2 were included, the significance of these findings could not be proven from our data.
Different from Taylor et al., the focus of the current study was to assess low histological grades of rejection without acute cardiac function compromise but with the risk to trigger CAV in the long-term. Thus, the original version of the histological grading of EMB was used, and, indeed, significant differences for both CMR parameters ER and gRE for patients with histological grades of rejection ≥1B were found.
This underlines the potential of CMR as a screening tool for early sub-clinical ACR also.
The diagnostic value of gRE may be diminished by the high inter- and intra-observer variability found in our study. This could be due to a different choice of slice to be evaluated or different selection of the reference muscle. On the other hand, cardiac T2-imaging as used for the measurement of ER has also been challenged by technical and pathophysiological considerations as well as a lack of scientific validation [34]. The currently used sequences may suffer from slow flow artefacts in the vicinity of hypokinetic myocardium. Additionally, the contrast-to-noise ratio for the detection of edema is typically quite low [35].
A promising method to improve diagnostic accuracy and decrease the variability is the use of quantitative T1- or T2-mapping, which has shown low variability in small studies [36, 37]. First, results of a study with 53 HTx patients showed a significant elevation of myocardial T2-values in patients with rejection [31]. Yet only four patients were included due to biologically proven acute rejection (histological grade of 2R or higher), whereas four other patients had assumed hemodynamic or humoral rejection. Further studies with a larger number of patients are needed to clarify the value of T2-mapping alone and in combination with other established parameters such as gRE or LGE.
The presence of LGE did not correlate with histological grade in our findings. Nor did the addition of LGE improve diagnostic performance. These findings are consistent with previously published results [18], and may be explained by the small size of areas of myocardial necrosis in ACR.
Additionally, myocardial fibrosis may also occur due to other causes different from ACR such as perioperative ischemia or repeated EMB [2]. An analysis of the specific pattern of LGE may increase its usefulness and has been shown to correlate with the presence of transplant coronary artery vasculopathy [38], which was not in the focus of our present study. Furthermore, the myocardial fibrosis in ACR might be diffuse and might be therefore not detectable by simple “eye-balling” like in this study.
Though our data pool is larger than in most other studies with 146 examinations from 73 patients, it is limited by the small number of positive EMB results. Because of the highly efficacious immunosuppressive therapy, biopsy-proven acute rejection of histological grades 2 and higher was rare among the included patients. A larger, multi-centre trial may be necessary to acquire a database with a larger number of cases.
Our study aimed to detect low grade ACR, which is sub-clinical in most cases [12]. In accordance with this, we did not find any significant difference in cardiac function parameters between patients with and without ACR. However, clinical information was not assessed for the current study, so it cannot be ruled out that a certain number of patients in our group with therapeutically relevant rejection exhibited clinical symptoms. This issue could be addressed in future studies by dedicated analysis of patient groups with our without clinical signs of rejection.
A different approach for the detection of ACR is the measurement of biomarkers derived from blood samples. Studies have shown promising results for the use of high sensitivity troponin assays [39, 40], brain natriuretic peptide [41], or genomic profiling [13, 42]. These methods could provide an even simpler and more readily available tool to monitor patients after HTx. However, other than CMR, they do not provide additional morphological or functional information. More data are needed to assess the potential of biomarkers alone or in combination with imaging techniques such as CMR.
In conclusion, our study demonstrated that a multi-sequential CMR examination has the potential of a non-invasive tool for exclusion of sub-clinical ACR in HTx patients with comparable results to CMR in myocarditis patients. Yet, the diagnostic performance on the whole is limited by rather poor specificity and, therefore, limited PPV. A decision to change the immunosuppressive therapy, therefore, cannot be made based on CMR results alone, but in combination with clinical information. Thus, CMR might be used instead of surveillance EMB, with positive CMR results leading to further clarification by clinical or laboratory findings and EMB. Secondly, CMR could be employed to monitor patients with low grade ACR over time.
Larger studies incorporating new techniques such as T1- and T2- mapping may hold the promise to overcome the present limitations and further establish CMR for the routine diagnostic workup of HTx patients until a completely non-invasive diagnostic management of patients after HTx may become possible.
Acknowledgments
The scientific guarantor of this publication is Prof. Dr. Matthias Gutberlet, Department of Diagnostic and Interventional Radiology, University of Leipzig - Heart Centre, Leipzig. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic study, performed at one institution.
Abbreviations
- ACR
Acute cellular rejection
- CMR
Cardiac magnetic resonance imaging
- EMB
Endomyocardial biopsy
- ER
Edema ratio
- gRE
Global relative enhancement
- ISHLT
International Society for Heart and Lung Transplantation
- HTx
Heart transplantation
- LV-EDV
Left-ventricular end-diastolic volume
- LV-EF
Left-ventricular ejection fraction
- LV-ESV
Left-ventricular end-systolic volume
- LGE
Late gadolinium enhancement
- PPV
Positive predictive value
- NPV
Negative predictive value
- STIR
Short tau inversion recovery
- TE
Echo time
- TR
Repetition time
References
- 1.Baraldi-Junkins C, Levin HR, Kasper EK, Rayburn BK, Herskowitz A, Baughman KL. Complications of endomyocardial biopsy in heart transplant patients. J Heart Lung Transplant. 1993;12:63–67. [PubMed] [Google Scholar]
- 2.Spiegelhalter DJ, Stovin PG. An analysis of repeated biopsies following cardiac transplantation. Stat Med. 1983;2:33–40. doi: 10.1002/sim.4780020105. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Raichlin E, Edwards BS, Kremers WK, et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant. 2009;28:320–327. doi: 10.1016/j.healun.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Brunner-La Rocca HP, Schneider J, Kunzli A, Turina M, Kiowski W. Cardiac allograft rejection late after transplantation is a risk factor for graft coronary artery disease. Transplantation. 1998;65:538–543. doi: 10.1097/00007890-199802270-00015. [DOI] [PubMed] [Google Scholar]
- 7.Mehra MR, Ventura HO, Chambers RB, Ramireddy K, Smart FW, Stapleton DD. The prognostic impact of immunosuppression and cellular rejection on cardiac allograft vasculopathy: Time for a reappraisal. J Heart Lung Transplant. 1997;16:743–751. [PubMed] [Google Scholar]
- 8.Yamani MH, Tuzcu EM, Starling RC, et al. Computerized scoring of histopathology for predicting coronary vasculopathy, validated by intravascular ultrasound. J Heart Lung Transplant. 2002;21:850–859. doi: 10.1016/S1053-2498(02)00415-1. [DOI] [PubMed] [Google Scholar]
- 9.Billingham ME. Histopathology of graft coronary disease. J Heart Lung Transplant. 1992;11:S38–S44. [PubMed] [Google Scholar]
- 10.Moien-Afshari F, McManus BM, Laher I. Immunosuppression and transplant vascular disease: benefits and adverse effects. Pharmacol Ther. 2003;100:141–156. doi: 10.1016/j.pharmthera.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Caforio AL, Tona F, Fortina AB, et al. Immune and nonimmune predictors of cardiac allograft vasculopathy onset and severity: multivariate risk factor analysis and role of immunosuppression. Am J Transplant. 2004;4:962–970. doi: 10.1111/j.1600-6143.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6:1248–1256. doi: 10.1111/j.1600-6143.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 13.Holweg CT, Potena L, Luikart H, et al. Identification and classification of acute cardiac rejection by intragraft transcriptional profiling. Circulation. 2011;123(20):2236–2243. doi: 10.1161/CIRCULATIONAHA.109.913921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.CIR.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 15.Gutberlet M, Spors B, Thoma T, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246(2):401–409. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 16.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 17.Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Magn Reson. 2009;11:7. doi: 10.1186/1532-429X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor AJ, Vaddadi G, Pfluger H. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail. 2010;12:45–51. doi: 10.1093/eurjhf/hfp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 20.Smart FW, Young JB, Weilbaecher D, Kleiman NS, Wendt RE, 3rd, Johnston DL. Magnetic resonance imaging for assessment of tissue rejection after heterotopic heart transplantation. J Heart Lung Transplant. 1993;12:403–410. [PubMed] [Google Scholar]
- 21.Revel D, Chapelon C, Mathieu D, et al. Magnetic resonance imaging of human orthotopic heart transplantation: correlation with endomyocardial biopsy. J Heart Transplant. 1989;8:139–146. [PubMed] [Google Scholar]
- 22.Wisenberg G, Pflugfelder PW, Kostuk WJ, McKenzie FN, Prato FS. Diagnostic applicability of magnetic resonance imaging in assessing human cardiac allograft rejection. Am J Cardiol. 1987;60:130–136. doi: 10.1016/0002-9149(87)90999-4. [DOI] [PubMed] [Google Scholar]
- 23.Marie PY, Carteaux JP, Angioi M, et al. Detection and prediction of acute heart transplant rejection: preliminary results on the clinical use of a "black blood" magnetic resonance imaging sequence. Transplant Proc. 1998;30:1933–1935. doi: 10.1016/S0041-1345(98)00486-2. [DOI] [PubMed] [Google Scholar]
- 24.Lund G, Morin RL, Olivari MT, Ring WS. Serial myocardial T2 relaxation time measurements in normal subjects and heart transplant recipients. J Heart Transplant. 1988;7:274–279. [PubMed] [Google Scholar]
- 25.Marie PY, Angioi M, Carteaux JP. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol. 2001;37:825–831. doi: 10.1016/S0735-1097(00)01196-7. [DOI] [PubMed] [Google Scholar]
- 26.Mousseaux E, Farge D, Guillemain R. Assessing human cardiac allograft rejection using MRI with Gd-DOTA. J Comput Assist Tomogr. 1993;17:237–244. doi: 10.1097/00004728-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Doornbos J, Verwey H, Essed CE, Balk AH, de Roos A. MR imaging in assessment of cardiac transplant rejection in humans. J Comput Assist Tomogr. 1990;14(1):77–81. doi: 10.1097/00004728-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging. 2007;26:452–459. doi: 10.1002/jmri.21028. [DOI] [PubMed] [Google Scholar]
- 29.Almenar L, Igual B, Martinez-Dolz L. Utility of cardiac magnetic resonance imaging for the diagnosis of heart transplant rejection. Transplant Proc. 2003;35:19621964. doi: 10.1016/s0041-1345(03)00653-5. [DOI] [PubMed] [Google Scholar]
- 30.Ciliberto GR, Mascarello M, Gronda E, et al. Acute rejection after heart transplantation: noninvasive echocardiographic evaluation. J Am Coll Cardiol. 1994;23:1156–1161. doi: 10.1016/0735-1097(94)90605-X. [DOI] [PubMed] [Google Scholar]
- 31.Usman AA, Taimen K, Wasielewski M, et al. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging. 2012;5:782–790. doi: 10.1161/CIRCIMAGING.111.971101. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45(11):1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 33.Zagrosek A, Abdel-Aty H, Boyé P, et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging. 2009;2:131–138. doi: 10.1016/j.jcmg.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Friedrich MG, Kim HW, Kim RJ, Kramer CM. T2 weighted imaging to assess post-infarct myocardium at risk. JACC Cardiovasc Imaging. 2012;4(9):1014–1021. doi: 10.1016/j.jcmg.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 36.Yingchoncharoen T, Jellis C, Popovoc Z, Flamm S, Kwon D. Reproducibility of multiple T1 mapping techniques and to ECV quantification. J Cardiovasc Magn Reson. 2014;16(Suppl 1):P18. doi: 10.1186/1532-429X-16-S1-P18. [DOI] [Google Scholar]
- 37.Wassmuth R, Prothmann M, Utz W, et al. Variability and homogeneity of cardiovascular magnetic resonance myocardial T2-mapping in volunteers compared to patients with edema. J Cardiovasc Magn Reson. 2013;15:27. doi: 10.1186/1532-429X-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steen MD, Constanze M, Sonja R, et al. Prevalence of different gadolinium enhancement patterns in patients after heart transplantation. J Am Coll Cardiol. 2008;52(14):11607. doi: 10.1016/j.jacc.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 39.Garrido IP, García-Lara J, Pinar E, et al. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012;110(5):655–661. doi: 10.1016/j.amjcard.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 40.Muñoz-Esparza C, Garrido IP, Blanco R, et al. Usefulness of high sensitivity troponin T assay in detecting acute allograft rejection after heart transplantation. Rev Esp Cardiol. 2011;64(12):1109–1113. doi: 10.1016/j.recesp.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Wu AH, Johnson ML, Aaronson KD, Gordon D, Dyke DB, Koelling TM. Brain natriuretic peptide predicts serious cardiac allograft rejection independent of hemodynamic measurements. J Heart Lung Transplant. 2005;24(1):52–57. doi: 10.1016/j.healun.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Lin D, Hollander Z, Ng RT, et al. Whole blood genomic biomarkers of acute cardiac allograft rejection. J Heart Lung Transplant. 2009;28(9):927–935. doi: 10.1016/j.healun.2009.04.025. [DOI] [PubMed] [Google Scholar]