Figure 7.
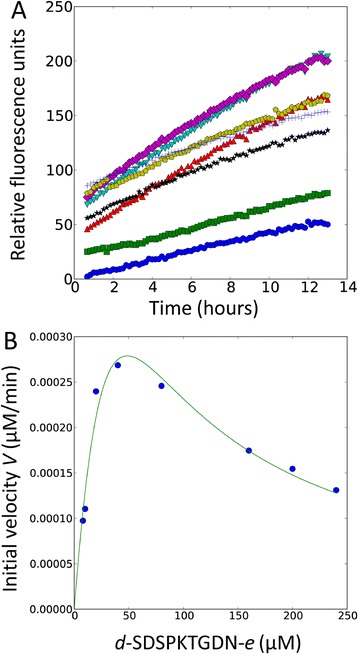
Kinetic parameters of SrtB
ΔN26
. In order to determine the in vitro kinetic parameters of SrtBΔN26 for the SPKTG and PPKTG motifs, we performed a kinetic analysis of the sortase-catalyzed hydrolysis reaction. A. Progress curves of the SrtBΔN26-catalyzed hydrolysis reactions at various concentrations of d-SDSPKTGDN-e [8 (blue ●), 10 (green ▪), 20 (red ▲), 40 (teal ▼), 80 (purple ♦), 160 (yellow  ), 200 (black ★), and 240 μM (blue +). The steady state rate (V) was determined by fitting the data to a linear function. B. Plot of V against the concentration of the peptide [S]. Nonlinear regression of these data fitted to Equation 1 resulted in a K
m of 74.7 ± 48.2 μM for d-SDSPKTGDN-e. SrtBΔN26 is subject to substrate inhibition at peptide concentrations > 30 μM, which is not expected to be physiologically relevant.
), 200 (black ★), and 240 μM (blue +). The steady state rate (V) was determined by fitting the data to a linear function. B. Plot of V against the concentration of the peptide [S]. Nonlinear regression of these data fitted to Equation 1 resulted in a K
m of 74.7 ± 48.2 μM for d-SDSPKTGDN-e. SrtBΔN26 is subject to substrate inhibition at peptide concentrations > 30 μM, which is not expected to be physiologically relevant.
