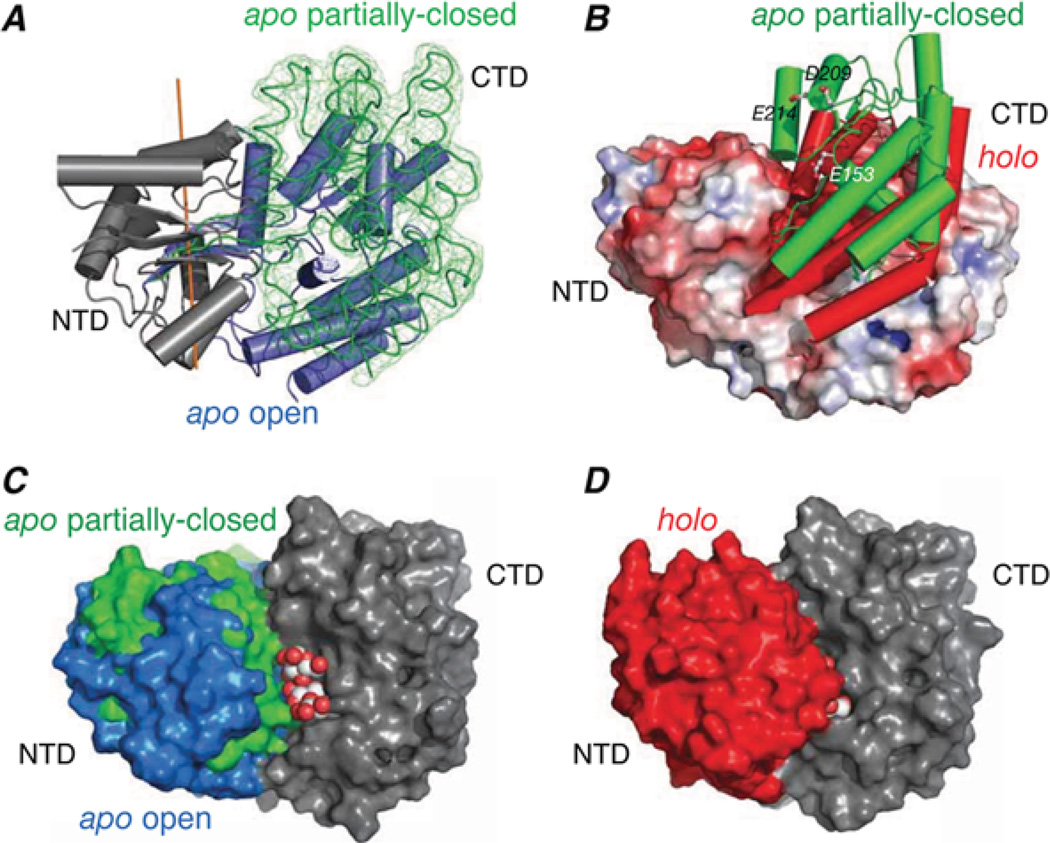
Figure 4. Structure of sparsely populated partially closed apo state of MBP derived from PRE measurements.
(A) Superimposition of the major open (blue cylinders [25]) and minor partially closed (green trace [28]) states of apo-MBP with the N-terminal domains (grey) of the two species superimposed. The reweighted atomic probability map for the backbone heavy atoms of the C-terminal domain in the partially closed state is displayed as a green mesh plotted at a threshold of 20 %. (B) Comparison of the C-terminal domain orientation in the partially closed form of apo-MBP (green cylinders) and holo-MBP (red cylinders [26]) with the apo open state shown as a molecular surface colour-coded according to electrostatic potential. (C) Molecular surface representation of the major open and minor partially closed states of apo-MBP best-fitted to the C-terminal domain (CTD; grey) with the N-terminal domain (NTD) displayed in blue and green respectively. A space-filling representation of maltotriose is modelled bound to the C-terminal domain. (E) Holo-MBP shown in the same view as in (C) with the N-terminal domain in red and the C-terminal domain in grey; the substrate is buried in holo-MBP and is barely visible. Adapted from Tang, C., Schwieters, C.D. and Clore, G.M. (2007) Open-to-closed transition in apo maltose-binding protein observed by paramagnetic NMR. Nature 449, 1078–1082 with permission.