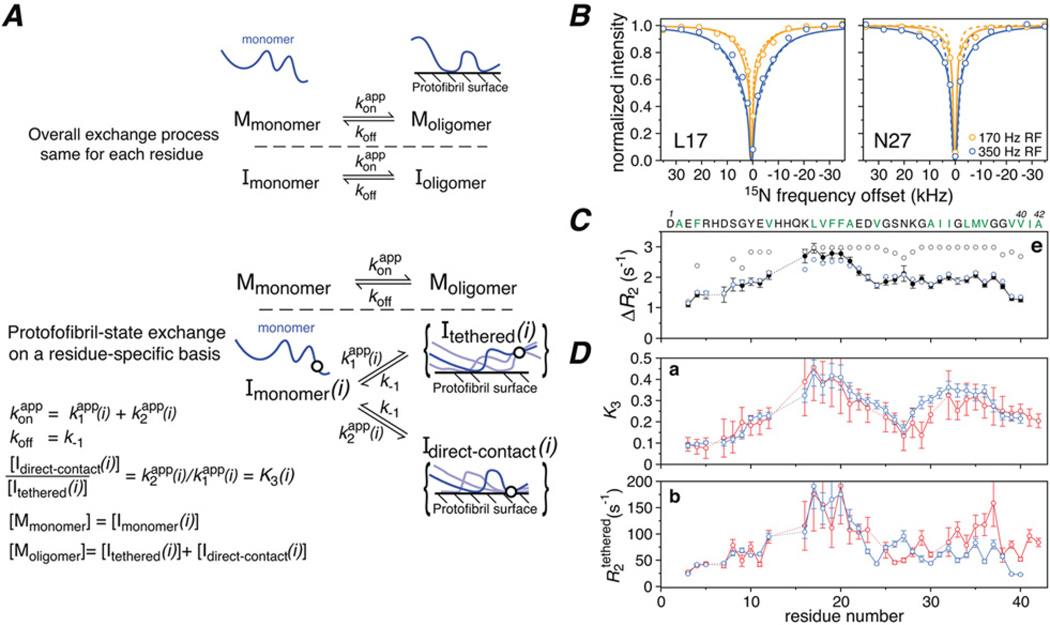
Figure 7. Exchange of Aβ monomer on the surface of large polydisperse protofibrils studied by 15N lifetime line broadening (Δ/R2) and 15N-DEST.
(A) Kinetic schemes for Aβ monomer exchange on the surface of amyloid protofibrils in which the protofibril-bound peptide (Moligomer) exists in only a single state (top), or a large ensemble of states such that each residue can be either tethered or in direct contact with the surface of the oligomer with K3(i)=k2 (app) (i)/ki (app) (i) (bottom). The circle in the diagrammatic representation of the states represents a single residue that is either tethered or in direct contact and for which three possible chain configurations are shown. (B) 15N-DEST profiles for Leu17 and Asn27 at two radiofrequency (RF) fields (170 Hz, orange; 350 Hz, blue) with the experimental points shown as circles, and the best-fit curves obtained with the single state or ensemble of states models shown as dashed and continuous lines respectively. (C) Comparison of the experimental 15N-ΔR2 profile (black closed circles) with the calculated profiles obtained with the single state (grey open circles) and ensemble of states (blue open circles) models. (D) Profiles for the residue-specific partition coefficient K3 (given by the ratio of direct contact to tethered states; see A, bottom panel) and 15N-R2 values for the tethered states derived from the fits to the experimental ΔR2 and DEST data. Adapted from Fawzi, N.L., Ying, J., Ghirlando, R., Torchia, D.A. and Clore, G.M. (2011) Atomic-resolution dynamics on the surface of amyloid-^ protofibrils probed by solution NMR. Nature 480, 268–272 with permission.