Abstract
Objectives: Acupuncture has been suggested as a treatment for spasticity in patients with stroke. The available literature was reviewed in an effort to assess its efficacy in this situation.
Methods: Randomized trials assessing the effects of acupuncture for the treatment of spasticity after stroke were identified by searching the Cochrane Library, PubMed, ProQuest, EBSCOhost, SCOPUS, CINAHL, EMBASE, Alternative Medicine Database, and Chinese and Korean medical literature databases. Two reviewers independently extracted data on study characteristics, patient characteristics, and spasticity outcomes.
Results: Eight trials with 399 patients met all the inclusion criteria. Compared with controls without acupuncture, acupuncture had no effect on improving clinical outcomes (as measured by validated instruments such as the Modified Ashworth Scale) or physiologic outcomes (assessed by measures such as the H-reflex/M-response [H/M] ratio at the end of the treatment period). H/M ratios did decrease significantly immediately after the first acupuncture treatment. Methodologic quality of all evaluated trials was considered inadequate.
Conclusions: The effect of acupuncture for spasticity in patients with stroke remains uncertain, primarily because of the poor quality of the available studies. Larger and more methodologically sound trials are needed to definitively confirm or refute any effect of acupuncture as a treatment for spasticity after stroke.
Introduction
Spasticity has been defined as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from the hyperexcitability of the stretch reflex.1,2 It is a characteristic feature of upper motor neuron syndromes, such as stroke, and is a potential obstacle to improvements in motor control and functional ability.3 Spasticity can cause difficulties in walking and activities of daily living, such as dressing and bathing.4 It may interfere with voluntary motor functions in patients with residual muscle power.5 It may also cause limbs to become fixed or frozen in an uncomfortable position. In some patients, severe spasticity causes pain, discomfort, and sleep disturbances.6 Accordingly, controlling spasticity is a major goal in rehabilitation after stroke syndromes.
Different treatments, such as physical therapy, orthotics, oral and intramuscular drugs, and surgical interventions, have been used to reduce spasticity. However, every conventional approach has its limitations,7 and no satisfactory long-lasting treatment for spastic muscles has yet been discovered. Thus, it is important to evaluate the effectiveness of “nonconventional” interventions, such as acupuncture.
Acupuncture is a relatively simple, inexpensive, and safe treatment modality.8 It has been used to improve motor, sensation, speech, and other neurologic functions in Chinese and Korean patients after stroke and may reduce spasticity in patients with stroke.9–12 These reported findings led us to investigate the effects of acupuncture in stroke patients with spasticity.
Most previous studies in this area have provided limited objective evidence in describing acupuncture's beneficial effects in terms of a reduction in spasticity after stroke. Although some meta-analyses have assessed the efficacy of acupuncture in stroke rehabilitation,13–17 no published systematic review of acupuncture has specifically focused on spasticity in stroke rehabilitation.
Materials and Methods
This study was exempt from institutional review board review because no human participants were involved.
Data sources
A literature search of English-language journals was performed using the Cochrane Library, PubMed, ProQuest, EBSCOhost, SCOPUS, CINAHL, EMBASE, and Alternative Medicine Database by two reviewers. Two authors reviewed Chinese-language journals by using the China Academic Journal Full-text Database by two reviewers. Two reviewers also searched Korean-language journals using the KoreaMed, RISS, KISTI, and DBpia databases. The literature review covered the period between January 1990 and August 2009. The key words used for the search were “acupuncture/electroacupuncture,” “stroke/CVA/cerebrovascular accident/cerebral infarction/intracerebral hemorrhage/ICH/cerebral embolism,” and “spasticity/muscle hypertonia.” Additionally, the references from all the identified studies and reviews were hand-searched for any other studies that might not have been identified by the electronic searches.
Selection
Two reviewers for the English, Chinese, and Korean literature each independently reviewed the trials for inclusion. In cases of disagreement between the two review authors, a third author reviewed the information to decide whether to include the study. The inclusion criteria were (1) randomized controlled clinical trials (RCTs) or quasi-randomized controlled trials; (2) trials comparing acupuncture of any kind with no acupuncture (or sham acupuncture); (3) patients with stroke (cerebral infarction, intracerebral hemorrhage, cerebral embolism, or unclassified stroke); (4) condition diagnosed clinically and/or by computed tomography or magnetic resonance imaging; and (5) spasticity measures.
Trials in which the acupuncture treatment did not involve needling, such as acupressure or laser acupuncture, and trials in which the control group underwent any kind of acupuncture were excluded. Furthermore, trials limited to patients with subarachnoid hemorrhage or subdural hematoma were excluded. The full text of the article was retrieved if there was any doubt about whether an article should be excluded.
Methodologic quality assessment
The Jadad scale was used to measure the quality of the included studies.18 This validated scale includes the following criteria: method of randomization, double-blinding, and reporting of withdrawals and dropouts. Because quality scales have their limitations, the individual components of study quality were evaluated for each included randomized controlled trial, including the concealment of treatment allocation, assessor blinding, intention-to-treat analysis, dropouts, and sample size. This quality assessment was performed by two reviewers independently, with disagreements reported to and resolved by a third author.
Data abstraction
Data were extracted independently from the included studies by the same two reviewers who were responsible for the literature searches. Once extraction was completed, any disagreement on data extraction and evaluation was resolved through discussion with or by a third party if necessary. Recorded data included study characteristics, patient characteristics, and outcomes. For the primary outcome of spasticity, evaluation measures included the Modified Ashworth Scale (MAS)19 and the H-reflex/M-response ratio (H/M ratio).20 Additionally, motor impairment measures, such as the Fugl-Meyer Assessment-Motor score (FMAM),21 and disability measures, such as the Barthel Index (BI)22 and Functional Independence Measure (FIM),23 were included as secondary outcome measures. All the extracted data were crosschecked, and differences were corrected by conferring with a third party, if necessary.
Data analysis
MIX software, version 2.0 (BIOSTATXL, Mountain View, CA) was used to calculate treatment effects across trials. Heterogeneity among trial results was tested using a standard chi-square test, with a threshold value of p<0.05. Overall comparisons were made between acupuncture and controls.
The standard deviation of the changes in outcome measures for each group could not be calculated because they were not always reported. Consequently, as an alternative method, the comparison of final measurements was used. In theory, this estimates the same quantity as the comparison of changes from the baseline in a randomized trial.24
In subgroup analysis, the plan was to compare effects in patients with different stroke causes, time to start of treatment, and spasticity severity at baseline, if appropriate data were available. Similarly, if appropriate data were available, a sensitivity analysis to assess the effects of including only those trials with double-blinding, with adequate concealment of randomization, and published in a language other than Chinese was planned. Finally, examination of any potential publication bias using a funnel plot was planned if sufficient RCTs were identified.25,26
Results
Qualitative findings
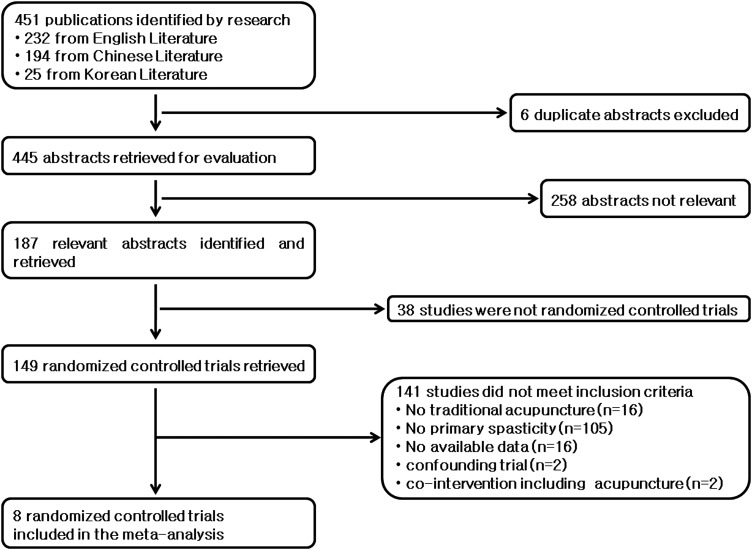
Details of the literature search are shown in Figure 1. Through the search, 232 English-language papers, 194 Chinese-language papers, and 25 Korean-language papers were identified. The 451 abstracts were scanned first and 187 full articles that were potentially relevant were reviewed further. Of the 187 studies remaining, 179 did not meet the inclusion criteria. Thirty-eight studies were excluded because they were not RCTs (24 were case reports, 11 were nonclinical trials, and 3 were nonrandomized studies). Twenty were excluded because they did not compare acupuncture of any kind with either no acupuncture or sham acupuncture. Moreover, 121 were excluded because they did not measure spasticity. This left eight RCTs with 399 patients meeting all the inclusion criteria. These eight studies were evaluated in this review and meta-analysis.
FIG. 1.
Details of the literature review and study identification for the systematic review.
The characteristics of the included studies are presented in Table 1. Of the eight included trials, one was conducted in Germany,27 six in China (including Hong Kong and Taiwan), and one in Korea.28–34 Seven trials did not describe their methods of randomization. One trial (60 patients) used a random-number table to allocate treatment.29 Adequate concealment of the randomization sequence from doctors treating the patients was reported in only one trial,29 which used sealed, opaque, and sequentially numbered envelops. One study (with 25 patients) comparing acupuncture with sham treatment was participant- and assessor-blinded,27 and one study was assessor-blinded.29 With the exception of one study,30 intention-to-treat analysis was not done and dropouts were not reported. On the whole, the methodologic quality of the eight RCTs was poor.
Table 1.
Summary of Studies According to Sample Size, Randomization Detail, Concealment of Allocation, Blinding, Intention to Treat, Drop Out, and Jadad Score
| Author, location (year) | Sample size (intervention/control), n (n/n) | Randomization detail | Concealment of allocation | Blinding | Intention to treat | Drop out | Jadad score |
|---|---|---|---|---|---|---|---|
| Fink et al., Germany (2004)27 | 25 (13/12) | Not stated | Unclear | Participants and assessor | Not stated | None | 3 |
| Gong et al., China (2008)28 | 63 (31/32) | Not stated | Unclear | None | Not stated | None | 1 |
| He and Zhang, China (2008)29 | 60 (20/20/20) | Random-number table | Central randomization | Assessor | Not stated | None | 2 |
| Lee et al., Korea (2007)30 | 20 (10/10) | Not stated | Unclear | None | Yes | 2 in control group | 2 |
| Li et al., China (2007)31 | 58 (29/29) | Not stated | Unclear | None | Not stated | None | 1 |
| Qu et al., China (2001)32 | 83 (41/42) | Not stated | Unclear | None | Not stated | None | 1 |
| Sun, China (2003)33 | 30 (20/10) | Not stated | Unclear | None | Not stated | None | 1 |
| Yan et al., China (2003)34 | 60 (30/30) | Not stated | Unclear | None | Not stated | None | 1 |
Five trials described the nature of the strokes.27,30,32–34 In total, 148 patients (three studies) received acupuncture starting less than 6 months after stroke onset.31,33,34 Another 85 patients (two studies) began acupuncture more than 6 months after stroke onset (Table 2).27,29
Table 2.
Summary of Studies According to Stroke Nature, Time from Stroke Onset, Inclusion, and Exclusion
| Author, location (year) | Stroke nature | Time from stroke onset | Inclusion | Exclusion |
|---|---|---|---|---|
| Fink et al., Germany (2004)27 | 14 infarction, 8 hemorrhage, 3 other causes | 7–180 mo | MAS ≥1 in the ankle joint | Anticoagulation, pregnancy, history of epileptic seizures, acute or chronic infectious diseases, and autoimmune diseases |
| Gong et al., China (2008)28 | Not stated | 37.8±8.8 d in acupuncture /35.6±9.2 d in control | (1) Stroke diagnosed by CT or MRI; (2) first stroke or recurrent stroke without previous neurologic deficit; (3) Brunnstrom stage ≥II | (1) Subarachnoid hemorrhage, progressive stroke; (2) deterioration of stroke; (3) unable to receive rehabilitation because of recent seizure; (4) deconditioning state due to major organ problem; (5) cognitive or communication problem |
| He and Zhang, China (2008)29 | Not stated | 21–58 d | (1) Brunnstrom stage: II–IV; (2) spasticity in lower limbs; (3) age <75 y, stroke diagnosed by CT or MRI; (4) stroke onset <3 mo, stable recovery, cognitive and communication function to participate in the therapy; (5) no experience with acupuncture or rehabilitation | (1) Age >76 y, able to receive treatment only at home; (2) stroke onset >3 mo; (3) difficulties with consciousness, cognition, or communication; (4) unable to participate in experiment because of medical problem; (5) flexor spasticity in lower limb; (6) rheumatism, fracture, trauma, joint contracture |
| Lee et al., Korea (2007)30 | 7 infarction, 11 hemorrhage | Not stated | Upper-limb spasticity | Patients receiving Oriental medical treatment |
| Li et al., China (2007)31 | Not stated | 2–6 mo | MAS >2, stable medical state | Difficulties with cognition or communication |
| Qu et al., China (2001)32 | 40 infarction, 13 hemorrhage, 30 others | 1–4 y | (1) increase muscle tone and 2) grasp power ≥grade III | Severe heart problem |
| Sun, China (2003)33 | 16 infarction, 14 hemorrhage | <6 mo | (1) stroke onset <6 mo and (2) Brunnstrom stage II–V | 1) premorbid motor weakness 2) complication of hypertension, coronary artery disease, renal dysfunction, pulmonary infection, severe diabetes mellitus, and psychologic history 3) difficulties with consciousness, cognition, and vision 4) taking antispastic medication recently |
| Yan et al., China (2003)34 | 35 infarction, 25 hemorrhage | 67.2±21.1 d in acupuncture/ 66.5±20.4 d in control | Increased muscle tone in limbs | Not stated |
MAS, Modified Ashworth Scale; CT, computed tomography; MRI, magnetic resonance imaging.
Seven trials compared acupuncture plus conventional stroke rehabilitation (CSR) with CSR alone,28–34 and one trial compared acupuncture with sham treatment.27 CSR refers to conventional modalities used in stroke rehabilitation, such as physiotherapy, occupational therapy, speech therapy, and skilled medical and nursing care.15 Among the trials included, one compared three arms, which were CSR plus low-frequency electrical acupuncture, high-frequency electrical acupuncture, and CSR alone.29 The actual acupuncture intervention varied across considerably the trials. Electric acupuncture was performed in five trials (284 patients),28–32 and manual acupuncture was performed in three trials (115 patients).27,33,34 All of the eight studies described the acupuncture points used in detail, but the selected points varied in both number of points stimulated and location of these sites. The most commonly used points were large intestine 4 (LI4), large intestine 10 (LI10), large intestine 11 (LI11), large intestine 15 (LI15), and stomach 36 (ST36). The total period of acupuncture treatment ranged from 2 weeks30 to 2 months.31 The number of treatment sessions varied from 827 to 60,31 and the frequency of treatment ranged between twice per week27 to once per day.28–34 The needle retention time ranged from 2030,32 to 45 minutes (Table 3).34 The most commonly reported primary outcome was MAS, and the most commonly used secondary outcomes was FMAM (Table 4).
Table 3.
Summary of Studies According to Acupuncture Technique, Details of Acupuncture, Control, and Cointervention
| Author, location (year) | Acupuncture technique | Details of acupuncture (acupoint, frequency, duration) | Control | Cointervention |
|---|---|---|---|---|
| Fink et al., Germany ( 2004)27 | Manual acupuncture | >10 acupoints (mainly LU9, LR3, LI4, LI10, LU9, ST36, SP9, GB34, GB39, GV20), 30 min twice per week for 4 wk | Sham acupuncture | None |
| Gong et al., China (2008)28 | Electrical acupuncture | 1 acupoint (ST36), 50 Hz, 5 times per week for 6 wk | No acupuncture | Physical and occupational therapy |
| He and Zhang, China (2008)29 | Electrical acupuncture | 7 acupoints (ST3, LR8, LR9, ST36, ST40, GB39, LR3), 100 Hz (high-frequency group)/2 Hz (low-frequency group), 30 min once per day for 30 d | No acupuncture | Physical therapy |
| Lee et al., Korea (2007)30 | Electrical acupuncture | 4 acupoints (PC3, PC2, PC6, LU5), 60 Hz, 20 min 5 times per week for 2 wk | No acupuncture | Conventional stroke rehabilitation |
| Li et al., China (2007)31 | Electrical acupuncture | 5 acupoints ( LI15, LI14, LI11, TE5, LI4), 1–100 Hz, 25 min once per day for 2 mo | No acupuncture | Physical therapy |
| Qu et al., China (2001)32 | Electrical acupuncture | 8 acupoints (LI15, LI11, LI4, LI10, ST32, ST34, ST36, ST41), 20 min, once per day for 30 d | No acupuncture | Physical therapy |
| Sun, China (2003)33 | Manual acupuncture | 7 acupoints (BL, DU, SI, SJ, LI, ST36, etc.), once per day for 30 d, including 3 d of rest | No acupuncture | Physical therapy |
| Yan et al., China (2003)34 | Manual acupuncture | 14 acupoints (jian qian, PC1, LU5, HT3, LU7, LI4, PC7, ST31, ST32, SP10, ST36, GB34, SP6), 45 min once per day for 24–48 d | No acupuncture | Physical therapy |
Table 4.
Summary of Studies According to Outcome Measure, Assessed Time, and Results
| Author, location (year) | Outcome measure | Assessed at: | Results |
|---|---|---|---|
| Fink et al., Germany ( 2004)27 | MAS(L), H/M ratio | Baseline, after first treatment, 4 wk | MAS(L): no significant differences between groups in any of follow-up assessment H/M ratio: significantly lower in intervention group assessed only at 4 wk |
| Gong et al., China (2008)28 | H/M ratio | Baseline, 6 wk | H/M ratio: significantly lower in intervention group assessed at 6 wk |
| He and Zhang, China (2008)29 | FMAM(L) | Baseline, 30 d | FMAM(L): significantly higher in both intervention groups than in control group and significantly higher in high-frequency group than in low-frequency group assessed at 30 d |
| Lee et al., Korea (2007)30 | MAS(U), H/M ratio, FMAM(U) | Baseline, 2 h, 2 wk | MAS(U): no significant differences of changes between groups in any of follow-up assessment H/M ratio: significantly improved in intervention group assessed at 2 h and 2 wk FMAM(U): no significant differences in changes between groups in any of follow-up assessment |
| Li et al., China (2007)31 | MAS(U), MAS(L), BI | Baseline, 2 mo | MAS(U), MAS(L): significantly lower in intervention group assessed at 2 mo BI: significantly higher in intervention group assessed at 2 mo |
| Qu et al., China (2001)32 | MAS(U), MAS(L), BI | Baseline, 30 d | MAS(U), MAS(L): significantly lower in intervention group assessed at 30 d BI: significantly higher in intervention group assessed at 30 d |
| Sun, China (2003)33 | MAS(U), MAS(L), FMAM(U), FMAM(L) | Baseline, 30 d | MAS(U), MAS(L): significantly lower in intervention group assessed at 30d FMAM(U), FMAM(L): no significant differences between groups assessed at 30d |
| Yan et al., China (2003)34 | MAS(U), MAS(L), FMAM(U) FMAM(L) | Baseline, 24–48 d | MAS(U), MAS(L): significantly lower in intervention group at follow-up assessment FMAM(U), FMAM(L): significantly higher in intervention group at follow-up assessment |
MAS, Modified Ashworth Scale; L, in lower extremities; H/M ratio, H-reflex/M-response ratio; FMAM, Fugl-Meyer assessment-motor score; U, in upper extremities; BI, Barthel Index.
None of the included trials reported the acupuncturists' background, such as the duration of relevant training and the length of clinical experience and expertise, and none of the trials provided information on adverse events.
Quantitative findings
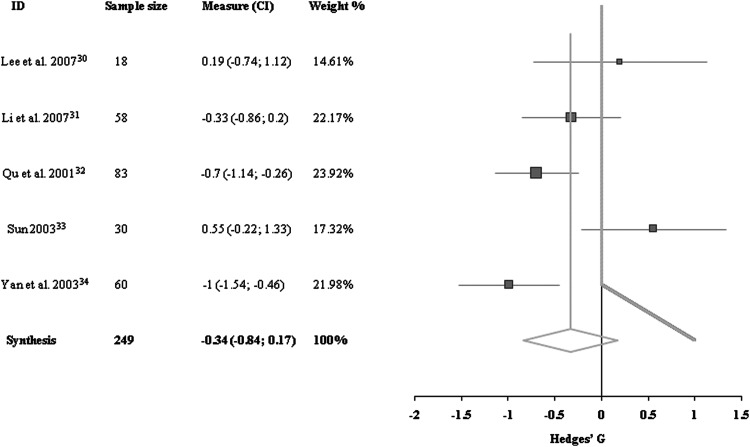
The two primary outcomes for spasticity included in this review were (1) MAS as a clinical measure, and (2) the H/M ratio as a neurophysiologic measure. Data for MAS outcome in the upper extremities were available for five trials, including a total of 249 patients.30–34 There was heterogeneity between these trials because of differences in stroke types and interventions. Looking at these five trials as a group, no significant difference was detected between acupuncture and control groups (−0.34 [95% confidence interval (CI), −0.84 to 0.17]; Fig. 2).
FIG. 2.
Comparisons of Modified Ashworth Scale of upper extremities at the end of treatment period between acupuncture group and control group. CI, confidence interval.
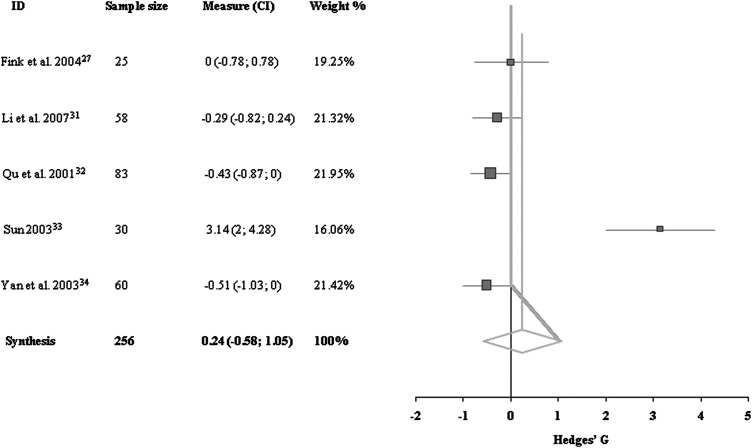
Data for MAS outcome of the lower extremities were available for five trials, with a total of 256 patients.27,31–34 There was heterogeneity among these trials due to differences in times of evaluation from stroke onset, stroke type, intervention, control groups used, and methodologic quality. No effect was detected on the improvement of MAS of the lower extremities after the acupuncture treatment (0.24 [95% CI, −0.58 to 1.05]; Fig. 3).
FIG. 3.
Comparisons of Modified Ashworth Scale of lower extremities at the end of treatment period between acupuncture group and control group.
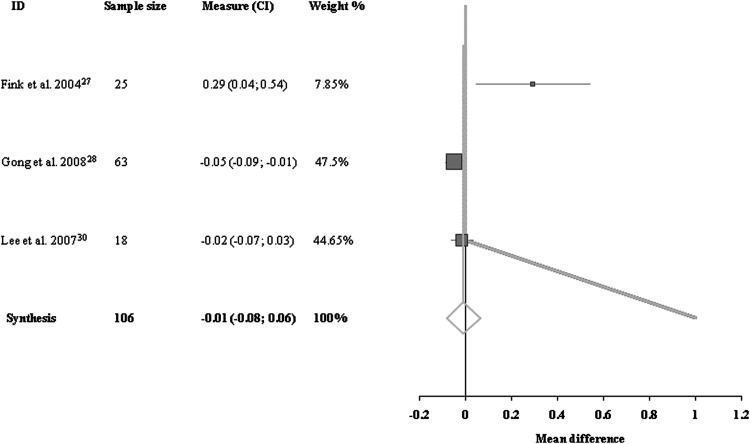
Data for H/M ratios were available for three trials with a total of 106 patients.27,28,30 There was heterogeneity among them, for reasons similar to those noted previously. There was no significant trend for H/M ratio in the acupuncture group compared with the control group (−0.01 [95% CI, −0.08 to 0.06]; Fig. 4).
FIG. 4.
Comparisons of H-reflex/M-response ratios at the end of treatment period between acupuncture group and control group.
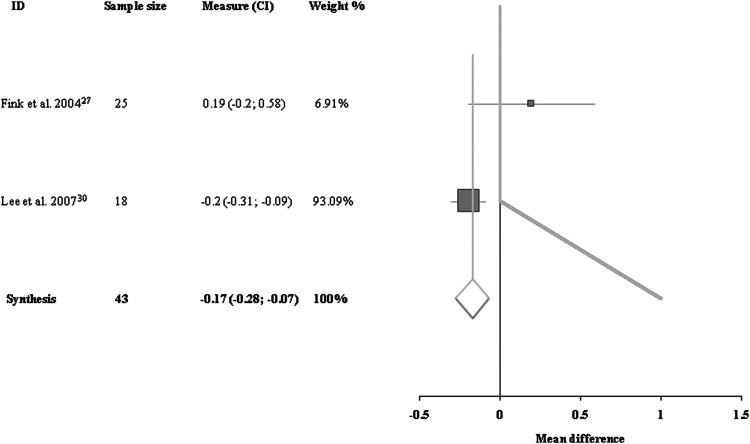
Data for H/M ratios immediately after the first treatment were available for two trials with a total of 43 patients.27,30 There was no heterogeneity among them. The immediate effect of acupuncture on the H/M ratio was significant compared with the control group (−0.17 [95% CI, −0.28 to −0.07]; Fig. 5).
FIG. 5.
Comparisons of H-reflex/M-response ratios at the end of the first treatment between acupuncture group and control group.
The secondary outcomes of spasticity included in this review are FMAM (as a motor recovery measure) and BI (as a disability measure). Three trials with 108 patients assessed FMAM of the upper extremities.30,32–34 There was significant heterogeneity among them, due mainly to differences in times of evaluation from stroke onset, stroke type, and intervention. Acupuncture had no positive effects above and beyond those of CSR on the motor recovery of the upper extremities on the basis of these studies (−6.50 [95% CI, −10.47 to −2.54]).
Three trials with 150 patients described FMAM of the lower extremities at the end of follow-up.29,32–34 There was significant heterogeneity among the three trials due to differences in time of evaluation from stroke onset, stroke type, intervention, and methodologic quality. Improvement in motor recovery in the lower extremities was higher in the acupuncture group (4.90 [95% CI, 4.26–5.53]).
Data for BI were available for two trials with 141 patients.31,32 There was no significant heterogeneity between them. Patients in the acupuncture group were less dependent for help in the activities of daily living than patients in the control groups; the difference was statistically significant (0.7 [95% CI, 0.27–0.95]).
It was not possible to conduct subgroup analysis based on stroke type, stroke lesion, time of starting acupuncture after onset of stroke, and spasticity severity because of limitations in the information available. It was also not possible to perform a meaningful sensitivity analysis review because of the small number of trials and the general poor quality of the trials. Additionally, a funnel plot could not be performed to check for publication bias because of the limited number of trials for each outcome.
Discussion
While knowledge of the neurophysiologic mechanisms leading to spasticity is limited,35 it is known that increased gamma-motor neuron activity, decreased inhibition by specific interneurons, and altered common interneuron activity increase alpha-motor neuron activity.36 Acupuncture may change the motor neuron activity and/or change synaptic transmission from muscle afferent terminals to spinal motor neurons, presumably mediated by presynaptic interneurons, and change intrinsic motor neuron properties, including membrane input resistance or membrane receptor responsiveness to released transmitters.37
Eight trials with a total of 399 patients were included in this review. Analysis of the trials appeared to show significant improvement in the motor recovery of the lower extremities and disability after acupuncture, but not in the spasticity measures. In the primary outcome measures of spasticity used, acupuncture had no additional benefit beyond CSR either in the clinical outcome, such as MAS, or in the neurophysiologic outcome, such as H/M ratios, at the end of the acupuncture treatment. However, the H/M ratios decreased significantly immediately after the first acupuncture treatment. The H/M ratio as an objective measure of the severity of spasticity reflects spinal alpha-motor neuron excitability.20 An improvement in H/M ratios is likely to be related to reduced excitability of alpha-motor neurons. Further work is needed to establish whether acupuncture definitely has this effect and to examine the possible mechanism responsible for acupuncture-induced inhibitory effects on alpha-motor neurons. However, the apparent immediate effect of acupuncture on spasticity detected in our review must be interpreted with caution. The result was based on data from two trials with a limited number of patients (in total, 45 patients), and one of the trials had poor methodologic quality in terms of the methods of randomization, allocation concealment, and blinding of assessment.
In the secondary outcomes assessed, motor recovery of the lower limbs improved significantly after acupuncture treatment. More improvement in disability score, such as the BI, was seen in the acupuncture group compared with the control group. These results are consistent with previous studies to some degree.15 However, no definite conclusion with respect to secondary outcomes can be reached because the quality of these studies was poor. Additionally, caution is needed in interpreting results related to motor recovery of the upper extremities. This result was associated mainly with Lee and colleagues' trial,30 which had more weight in the meta-analysis. Final FMAM and baseline FMAM scores in the intervention group were lower than each of them in the control group in Lee and colleagues' study.30 This contributed to the negative effect of acupuncture on FMAM of the upper extremity. As the design of Lee and colleagues' study30 included randomization, this final outcome was included in the meta-analysis. However, the ratio changes in FMAM in intervention group were significantly larger in Lee and colleagues' study.30
A strength of the present review is that it appears to be the first systematic review to examine the effects of acupuncture on spasticity after stroke. Although a few systematic reviews have evaluated the effects of acupuncture on stroke,13–17 no reported study has evaluated the effects of acupuncture on the treatment of spasticity after stroke. Because many trials had poor methodologic quality and had varying treatment protocols, the heterogeneity made it difficult to consider the trials as a single data set. However, it is meaningful to analyze and evaluate the effects of acupuncture on spasticity of stroke for the first time and give guidance about the design of future trials. For complementation, trials that met inclusion criteria were included and analyzed.
Another strength is that this review evaluated acupuncture's effects on the improvement in spasticity using both clinical and neurophysiologic spasticity measures. Clinical spasticity measures showed a similar trend to the neurophysiologic measures in this review. Finally, both primary outcomes and secondary outcomes (such as motor recovery and disability) were used to assess acupuncture's effects on spasticity.
The insufficient number of trials prohibited meaningful sensitivity analysis to assess how robust the results of this review might be. Smith and colleagues found that sensitivity analyses, based on blinding, reporting quality, validity score, and country of origin, showed a higher proportion of positive results for poor-quality studies than for those of higher quality in assessing the evidence for the effectiveness of acupuncture.38 With the available information, it was not possible to perform prespecified subgroup analysis comparing patients with different cause of stroke, stroke lesion, time to start treatment, and spasticity severity at baseline. This was due to the limited amount of data, the lack of universally available outcome measurements, or both. Furthermore, it was not possible to perform a funnel plot to assess the degree of publication bias in this review because of the limited numbers of trials available for each outcome. Although extensive searches for published materials were undertaken, the possibility that studies with negative findings remain unpublished cannot be excluded.
Misleading results may occur with acupuncture if the treatment schedules were inadequate or if it was done by unskilled practitioners. However, information on the experience and training of the acupuncturists who applied the treatments was not available in any of the included trials. The acupuncture techniques, the number of acupuncture points stimulated, the number and duration of sessions, and the intervention period also varied across the trials. Also, within some trials, the acupuncture points, the number of sessions, and the duration of sessions were individualized according to the practical conditions of each stroke patient. Because of the limited or incomplete description of the acupuncture treatments used in the included trials, it was difficult to evaluate whether the acupuncture treatment was valid. Additionally, only one trial meeting the inclusion criteria for this review used sham acupuncture. Because of this, it is possible that a placebo effect of acupuncture treatment existed. Although a recent systematic review suggested that placebos had no significant effects on outcomes in clinical trials,39 placebos should be used where they are acceptable. Finally, the lack of consistency in outcome measures available was an obstacle to assessing the effect of acupuncture on spasticity after stroke in a systematic manner.
In future, acupuncture trials should be designed to overcome the limitations noted in the trials included in this review. In particular, they should (1) ensure adequate concealment of allocation, (2) ensure proper blinding of outcome and assessors, (3) use placebo or sham acupuncture as the control, (4) use standard validated spasticity outcome measurements, (5) have long-term follow-up, and (6) publish the results in a usable form to facilitate meta-analysis. Studies should also clearly define the modality of acupuncture used and include information on the acupuncture techniques/protocol, based on evidence or a consensus of experts (STRICTA).40,41 Adverse events critically assessed by standardized monitoring or an effective self-report system should also be described.
Conclusions
The results of this meta-analysis do not support that acupuncture has a beneficial effect on reducing spasticity in poststroke patients. However, results suggest that acupuncture may have an adjuvant effect on spasticity by modulating alpha-motor neuron activity. The widespread use of acupuncture, its potential for benefits in stroke spasticity with less severe adverse effects, its lower cost, and the insufficient quality of the available trials all argue for further research. Further well-designed trials are required to make any definitive conclusion about the effect of acupuncture in the treatment of spasticity after stroke. Specifically, large sham- or placebo-controlled trials are needed.
Acknowledgments
This research was supported by a grant (08-B-02) from the Korea National Rehabilitation Center Research Institute.
Author Disclosure Statement
No competing financial relationships exist.
References
- 1.Lance J. Symposium synopsis. In: Feldman R, Young R, Koella W, eds. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 1980: pp. 485–494 [Google Scholar]
- 2.Lamontagne A, Malouin F, Richards CL, et al. . Evaluation of reflex- and nonreflex-induced muscle resistance to stretch in adults with spinal cord injury using hand-held and isokinetic dynamometry. Phys Ther 1998;78:964–975 [DOI] [PubMed] [Google Scholar]
- 3.Bobath B. Adult Hemiplegia: Evaluation and Treatment. London: Butterworth Heinemann, 1990 [Google Scholar]
- 4.Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN. Intrathecal baclofen for spastic hypertonia from stroke. Stroke 2001;32:2099–2109 [DOI] [PubMed] [Google Scholar]
- 5.Mizrahi EM, Angel RW. Impairment of voluntary movement by spasticity. Ann Neurol 1979;5:494–495 [DOI] [PubMed] [Google Scholar]
- 6.Jagatsinh Y. Intrathecal baclofen: Its effect on symptoms and activities of daily living in severe spasticity due to spinal cord injuries: a pilot study. Indian J Orthop 2009;43:46–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilton AH. Management of spasticity in children with cerebral palsy. Semin Pediatr Neurol 2004;11:58–65 [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health. Acupuncture. NIH Consensus Statement. 1997. Online document at: http://consensus.nih.gov/1997/1997acupuncture107html.htm Accessed on August12, 2014
- 9.Guo Z, Zhou M, Chen X, Wang R. Acupuncture methods for hemiplegic spasm. J Tradit Chin Med 1997;17:284–288 [PubMed] [Google Scholar]
- 10.Wong AM, Su TY, Tang FT, et al. . Clinical trial of electrical acupuncture on hemiplegic stroke patients. Am J Phys Med Rehabil 1999;78:117–122 [DOI] [PubMed] [Google Scholar]
- 11.Zhao JG, Cao CH, Liu CZ, et al. . Effect of acupuncture treatment on spastic states of stroke patients. J Neurol Sci 2009;15:143–147 [DOI] [PubMed] [Google Scholar]
- 12.Sanner G. Acupuncture for the relief of painful muscle spasms in dystonic cerebral palsy. Dev Med Child Neurol 1981;23:544–546 [DOI] [PubMed] [Google Scholar]
- 13.Ernst E, White AR. Acupuncture as an adjuvant therapy in stroke rehabilitation? Wien Med Wochenschr 1996;146:556–558 [PubMed] [Google Scholar]
- 14.Park J, Hopwood V, White AR, Ernst E. Effectiveness of acupuncture for stroke: a systematic review. J Neurol 2001;248:558–563 [DOI] [PubMed] [Google Scholar]
- 15.Sze FK, Wong E, Or KK, et al. . Does acupuncture improve motor recovery after stroke? A meta-analysis of randomized controlled trials. Stroke 2002;33:2604–2619 [DOI] [PubMed] [Google Scholar]
- 16.Wu HM, Tang JL, Lin XP, et al. . Acupuncture for stroke rehabilitation. Cochrane Database Syst Rev 2006;19:CD004131. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SH, Liu M, Asplund K, Li L. Acupuncture for acute stroke. Cochrane Database Syst Rev 2005;18:CD003317. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–207 [DOI] [PubMed] [Google Scholar]
- 20.Joodaki MR, Olyaei GR, Bagheri H. The effects of electrical nerve stimulation of the lower extremity on H-reflex and F-wave parameters. Electromyogr Clin Neurophysiol 2001;41:23–28 [PubMed] [Google Scholar]
- 21.Fugl-Meyer AR, Jaasko L, Leyman I, et al. . The poststroke hemiplegic patient, I: a method for evaluation of physical performance. Scand J Rebabil Med 1975;7:13–31 [PubMed] [Google Scholar]
- 22.Sulter G, Steen C, Keyser JD. Use of the Barthel Index and Modified Rankin Scale in acute stroke trials. Stroke 1999;30:1538–1541 [DOI] [PubMed] [Google Scholar]
- 23.Hamilton BB, Granger CV, Sherwin FS, et al. . A uniform national data system for medical rehabilitation. In: Fuhrer MJ, ed: Rehabilitation Outcomes: Analysis and Measurement. Baltimore, MD: Brookes, 1987:137–147 [Google Scholar]
- 24.Higgins JPT, Green S/. Special topics in statistics: missing standard deviations. In: Higgins JPT, Deeks JJ, Altman DG, eds. Cochrane Handbook for Systematic Reviews of Interventions. Australia: Wiley-Blackwell, 2008: pp. 485–486 [Google Scholar]
- 25.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controll Clin Trials 1998;19:159–166 [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink M, Rollnik JD, Bijak M, et al. . Needle acupuncture in chronic poststroke leg spasticity. Arch Phys Med Rehabil 2004;85:667–672 [DOI] [PubMed] [Google Scholar]
- 28.Gong WJ, Zhang T, Cui LH, et al. . [Effect of electroacupuncture at Zusanli(ST36) on stroke patients in hemiplegic spasm period]. Capital China Rehabilitation Research Center Beijing. 2008;14:1165–1167 [Google Scholar]
- 29.He J, Zhang H. [Observation of surface electrmyography when treating spasm of lower limb after stroke with different frequency of electric acupuncture]. J Guangzhou Univ Chin Med 2008;2:CNKI:CDMD:100414 [Google Scholar]
- 30.Lee SW, Yun JM, Son JW, et al. . [The effect of electroacupuncture on upper-extremity spasticity of stroke patients]. Korean J Orient Int Med 2007;28:492–501 [Google Scholar]
- 31.Li L, Yang WX, Hu FJ, et al. . [Clinical effect of electric acupuncture and baclofen on recovery of spasticity]. Chin J Rehabil 2007;22:319–320 [Google Scholar]
- 32.Qu J, Mo T, Yang B. [The effect of electroacupuncture on spasm of hemiplegia after traumatic brain injury]. Modern Rehabil Med 2001;5:3.:114. [Google Scholar]
- 33.Sun H. [Skin needles treat on spasticiy of hemiplegia after stroke]. J Beijing Univ Chin Med 2003;2:CNKI:CDMD:072251 [Google Scholar]
- 34.Yan W, Huo W, Yin J. [Study of the effect of combined acupuncture and exercise on treatment of spasticity after stroke]. Chin J Cardiovasc Rehab Med 2003;12:370–371 [Google Scholar]
- 35.Price R, Bjornson KF, McLaughlin JF, Hays RM. Quantitative measurement of spasticity in children with cerebral palsy. Dev Med Child Neurol 1991;33:585–595 [DOI] [PubMed] [Google Scholar]
- 36.Milanov I. Examination of the segmental pathophysiological mechanisms of spasticity. Electromyogr Clin Neurophysiol 1994;34:73–79 [PubMed] [Google Scholar]
- 37.Lee JH, Beitz AJ. Electroacupuncture modifies the expression of c-fos in the spinal cord induced by noxious stimulation. Brain Res 1992;577:80–91 [DOI] [PubMed] [Google Scholar]
- 38.Smith LA, Moore OA, McQuay HJ, Moore A. Assessing the evidence of effectiveness of acupuncture for stroke rehabilitation: stepped assessment of likelihood of bias. 2002. Online document at: http://www.medicine.ox.ac.uk/bandolier/booth/alternat/acstroke.html Accessed on August12, 2014
- 39.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:1594–1602 [DOI] [PubMed] [Google Scholar]
- 40.MacPherson H, White A, Cummings M, Jobst K, Rose K, Niemtzow R. Standards for reporting interventions in controlled trials of acupuncture: the STRICTA recommendations. Complement Ther Med 2001;9:246–249 [DOI] [PubMed] [Google Scholar]
- 41.White AR, Filshie J, Cummings TM. Clinical trials of acupuncture: consensus recommendations for optimal treatment, sham controls and blinding. Complement Ther Med 2001;9:237–245 [DOI] [PubMed] [Google Scholar]