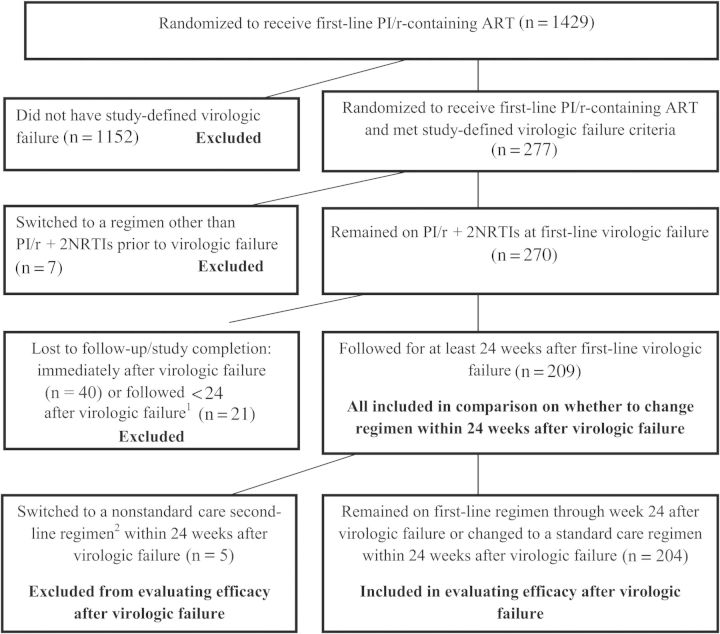
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram for inclusion and exclusion criteria. 1Among the 21 patients with some follow-up, 16 stayed on their first-line ritonavir-boosted protease inhibitor (PI/r)–based regimen; 13 of the 21 (62%) had HIV-1 RNA <400 copies/mL at their last available measurement after initial virologic failure. 2The 5 participants who changed to a nonstandard regimen included 3 who changed to an unboosted protease inhibitor–containing regimen and 2 who changed to a PI/r + nonnucleoside reverse transcriptase regimen. Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1; NRTI, nucleoside reverse transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitor.