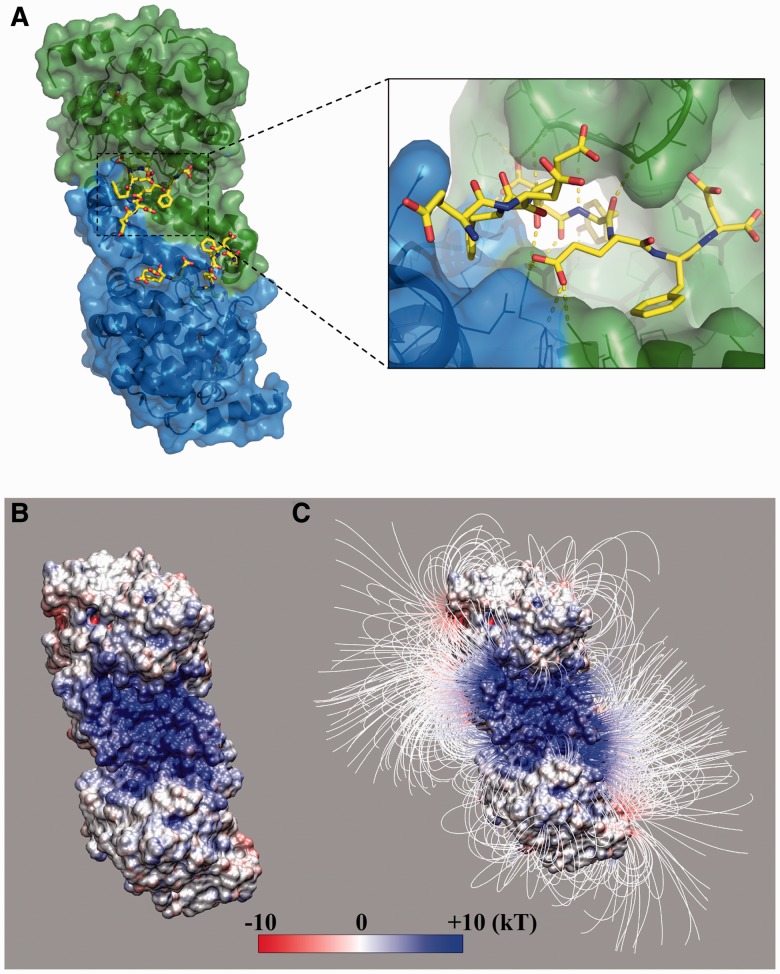
Fig. 1.
The structure of TPST-2 dimer. (A) The TPST-2 dimer (Teramoto et al., 2013) with bound substrate peptide (yellow) and PAPS analog. (B) Electrostatic potential and (C) field lines of the enzyme after removing the substrate peptide. The binding pocket has a high positive electrostatic potential, and hence, the peptides with net negative charge can be driven into the pocket by electrostatic interactions