FIGURE 1.
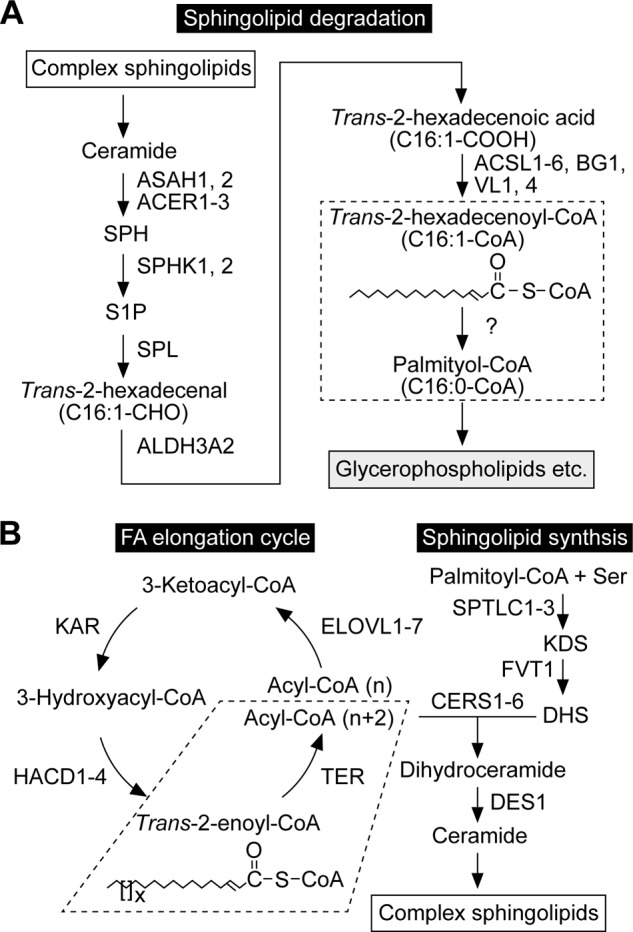
The pathways of sphingolipid degradation and synthesis. A, sphingolipid degradation pathway and involved enzymes. Complex sphingolipids are degraded to SPH by lysosomal degrading enzymes. After SPH is phosphorylated, the resulting S1P is metabolized to palmitoyl-CoA via the S1P metabolic pathway. In this pathway, S1P is first cleaved to trans-2-hexadecenal and phosphoethanolamine. Trans-2-hexadecenal is then oxidized to trans-2-hexadecenoic acid, followed by CoA addition. The produced trans-2-hexadecenoyl-CoA is finally saturated to palmitoyl-CoA by a trans-2-enoyl-CoA reductase (dashed box; subject of this study). The corresponding enzyme was unknown at the beginning of this study but identified as TER in the process. Palmitoyl-CoA is mainly active in glycerophospholipid synthesis, but may to some extent be metabolized to other lipids or degraded via β-oxidation. B, pathways of sphingolipid biosynthesis and FA elongation, and enzymes involved in each step. In the de novo sphingolipid biosynthetic pathway, palmitoyl-CoA and serine are condensed to 3-ketodihydrosphingosine (KDS), which is then reduced to DHS. DHS reacts with acyl-CoA to generate dihydroceramide. Acyl-CoA with ≥C18 is produced through FA elongation cycle. The last step of FA elongation cycle (dashed box) is catalyzed by the trans-2-enoyl-CoA reductase TER. The LCB moiety of dihydroceramide is converted from DHS to SPH by introducing a trans double bond between C4 and C5 to produce ceramide. Ceramide is converted to complex sphingolipids by receiving phosphocholine (sphingomyelin) or sugars (glycosphingolipids). The simplest glycosphingolipids are the monohexosylceramides glucosylceramide and galactosylceramide, which contain one glucose and one galactose residue, respectively. The addition of galactose to the glucose residue of glucosylceramide creates lactosylceramide. More than 100 glycosphingolipids originate from lactosylceramide, including globo-series Gb3 and Gb4 and ganglio-series GM3.
