FIGURE 1.
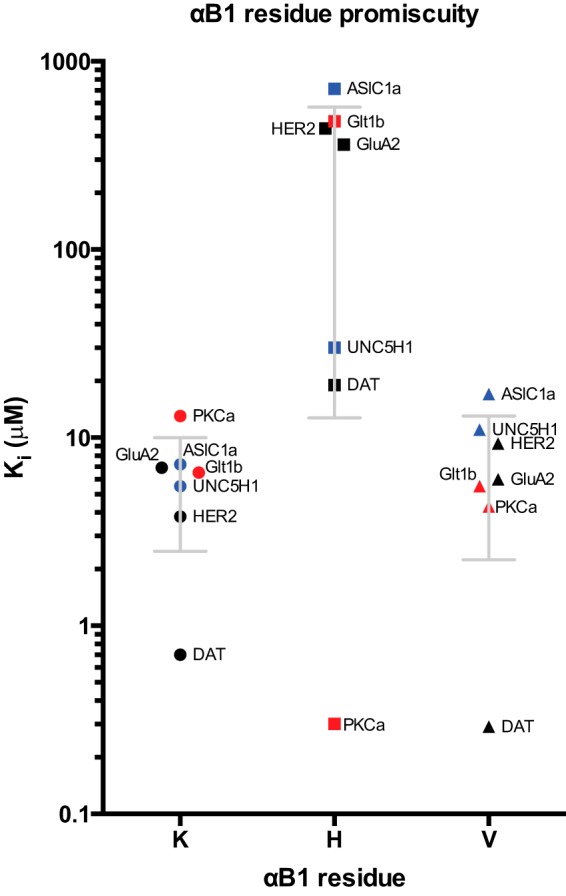
Affinity scatter profile (Ki values) of class-specific mutations in the αB1 position of the PICK1 PDZ domain. The effect of different residue in the αB1 position (x axis) was probed in a competition FP assay. Calculated Ki values are shown on the y- axis. For WT and K83V, OG DAT C13 is used as tracer. For K83H mutant, OG PKCα C13 is used as tracer. The K83H mutant disrupted the ligand binding promiscuity and switched the preference to class I ligands. The class II K83V mutant showed an almost preserved binding pattern, although the observed affinities for the class II and unclassified ligand were more dispersed with the lowest affinities for the unclassified ligands. Class I, II, and unclassified ligand peptides are indicated by red, black, and blue, respectively. See also Table 1.
