FIGURE 2.
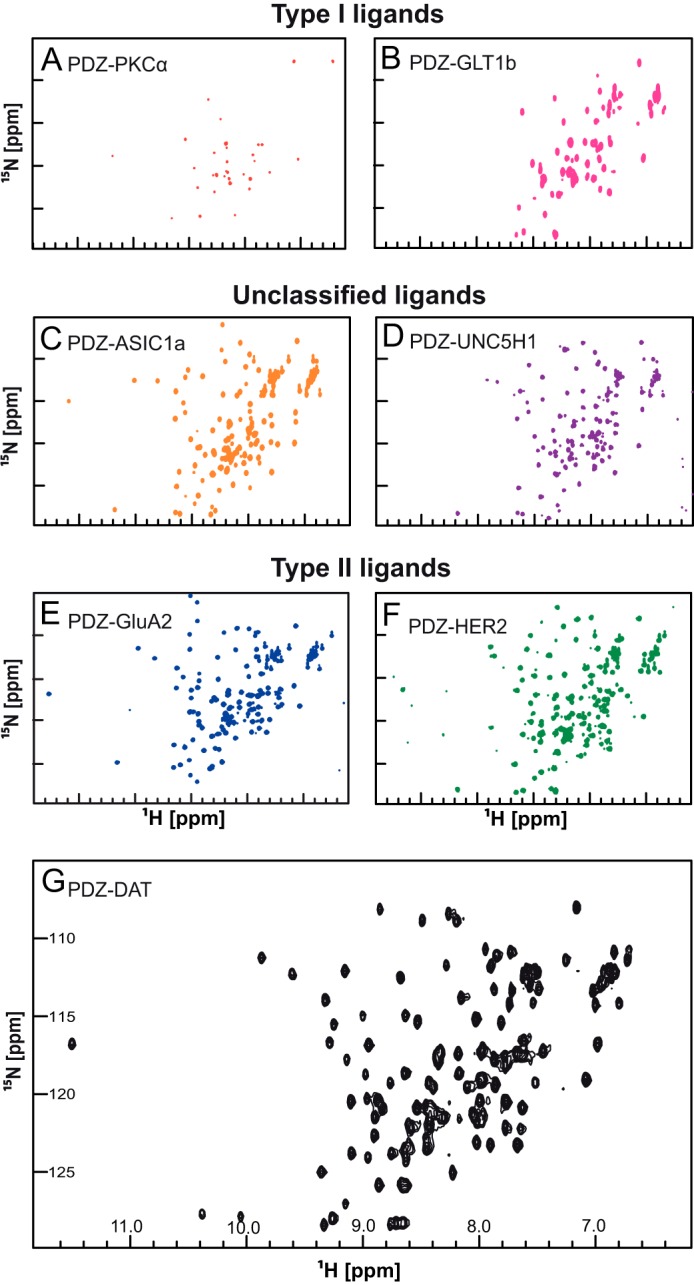
1H-15N HSQC NMR spectra of PICK1 PDZ ligand constructs. The 1H-15N-HSQC NMR experiment shows signals from all the backbone and side chain N-H correlations with 1H along the x axis and 15N along the y axis. Changes in the local environment surrounding a particular residue cause chemical shifts to deviate. Residues in a disordered protein have similar local environments causing the HSQC spectrum to exhibit low dispersion of chemical shifts as observed in this figure. Two class I ligand PDZ domain constructs, PKCα (A) and Glt1b (B) were bacterially expressed and purified. Both constructs showed low dispersion 1H-15N HSQC spectra indicating a low fraction of correctly folded PDZ domain protein. Aggregation was observed in both NMR samples. Fusion constructs with the C terminus of unclassified ligands, ASIC1a (C) and UNC5H1 (D), showed well dispersed HSQC spectra, although the ASIC1a HSQC spectrum was better resolved than the UNC5H1 HSQC spectrum. Three class II ligand, GluA2 (E), HER2 (F), and DAT (G), constructs showed well resolved HSQC spectra. All experiments were performed using 16 scans and 128 increments at 15 °C with an 800 MHz spectrometer.
